Apremilast (Monograph)
Brand name: Otezla
Drug class: Phosphodiesterase-4 Inhibitors, Miscellaneous
Introduction
Disease-modifying antirheumatic drug (DMARD); a selective phosphodiesterase type 4 (PDE4) inhibitor.
Uses for Apremilast
Psoriatic Arthritis
Management of active psoriatic arthritis in adults.
Various drugs and drug classes are used to treat psoriatic arthritis. Apremilast is considered an oral small molecule (OSM), which is one of several classes of disease-modifying treatments for this condition.
The American College of Rheumatology (ACR)/National Psoriasis Foundation guideline conditionally recommends the use of TNF blocking agents over OSMs in treatment-naive patients with active psoriatic arthritis; however, an OSM such as apremilast may be considered in treatment-naive patients without severe psoriasis or psoriatic arthritis, in those who prefer oral over parenteral therapy, and those with contraindications to TNF blocking therapy (e.g., CHF, previous serious infection, recurrent infections, demyelinating disease).
In patients not responding to treatment with an OSM, switching to another OSM is generally recommended over adding another OSM to the current regimen except in the case of apremilast since evidence of benefit with this drug is mostly with combination therapy. Switching to apremilast monotherapy may be considered instead of apremilast combination therapy if the patient has intolerable adverse events with the current OSM.
Recommendations for use and selection of disease-modifying therapies in psoriatic arthritis vary based on the presence of certain disease characteristics (e.g., psoriatic spondylitis/axial disease, enthesitis) and comorbidities (e.g., inflammatory bowel disease, diabetes).
Plaque Psoriasis
Management of plaque psoriasis in adults who are candidates for phototherapy or systemic therapy.
Treatment of pediatric patients ≥6 years of age weighing ≥20 kg with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Various drugs and drug classes are used to treat psoriasis including systemic biologic and nonbiologic therapies; apremilast is a nonbiologic option for treatment of this condition.
Guidelines generally recommend apremilast for treatment of adults with moderate to severe psoriasis; also may be used to augment efficacy of certain biologic agents (e.g., adalimumab, etanercept, infliximab, ustekinumab).
Recommendations for use and selection of psoriasis therapies vary based on patient age, disease characteristics (e.g., severity, location, presence of psoriatic arthritis), and comorbidities (e.g., inflammatory bowel disease).
Oral Ulcers Associated with Behçet Disease
Management of oral ulcers associated with Behçet disease in adults.
Treatment of Behçet disease is determined by involved organs, severity and duration of disease, age, gender, frequency of attacks, and patient preferences. The European Alliance of Associations for Rheumatology (EULAR) guidelines recommend topical treatments (such as steroids) and colchicine for mucocutaneous lesions. Apremilast can be considered as an alternative to colchicine in selected patients who have recurrent lesions despite colchicine use.
Apremilast Dosage and Administration
General
Pretreatment Screening
-
Evaluate renal function prior to starting apremilast; dosage reductions are recommended for severe renal impairment.
-
Weigh the risks and benefits of apremilast therapy in patients with a history of depression and/or suicidal thoughts or behaviors.
Patient Monitoring
-
Monitor patients for the potential development of serious hypersensitivity reactions including anaphylaxis and angioedema.
-
Monitor patients for diarrhea, nausea, and vomiting.
-
Monitor for emergence or worsening of depression, suicidal thoughts, or other mood changes.
-
Monitor weight regularly; consider discontinuing apremilast if unexplained or clinically significant weight loss occurs.
Administration
Oral Administration
Administer orally without regard to meals.
Swallow tablets intact. Do not crush, split, or chew.
Dosage
Pediatric Patients
Plaque Psoriasis
Oral
≥6 years of age weighing ≥20 kg: Recommended dosage based on body weight.
≥50 kg: Titrate as follows to reduce the risk of GI symptoms:
-
10 mg in the morning on day 1
-
10 mg in the morning and 10 mg in the evening on day 2
-
10 mg in the morning and 20 mg in the evening on day 3
-
20 mg in the morning and 20 mg in the evening on day 4
-
20 mg in the morning and 30 mg in the evening on day 5
Maintenance dosage (beginning on day 6): 30 mg twice daily.
20 to <50 kg: Titrate as follows to reduce the risk of GI symptoms:
-
10 mg in the morning on day 1
-
10 mg in the morning and 10 mg in the evening on day 2
-
10 mg in the morning and 20 mg in the evening on day 3
Maintenance dosage (beginning on day 4): 20 mg twice daily.
Adults
Psoriatic Arthritis
Oral
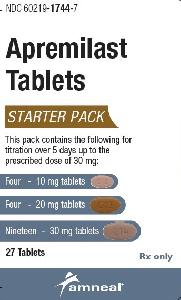
Initiate at a dosage of 10 mg daily and titrate as follows (over 5 days in increments of 10 mg daily) to reduce the risk of GI symptoms:
-
10 mg in the morning on day 1
-
10 mg in the morning and 10 mg in the evening on day 2
-
10 mg in the morning and 20 mg in the evening on day 3
-
20 mg in the morning and 20 mg in the evening on day 4
-
20 mg in the morning and 30 mg in the evening on day 5, and 30 mg twice daily thereafter
Maintenance dosage: 30 mg twice daily.
Plaque Psoriasis
Oral
Initiate at a dosage of 10 mg daily and titrate as follows (over 5 days in increments of 10 mg daily) to reduce the risk of GI symptoms:
-
10 mg in the morning on day 1
-
10 mg in the morning and 10 mg in the evening on day 2
-
10 mg in the morning and 20 mg in the evening on day 3
-
20 mg in the morning and 20 mg in the evening on day 4
-
20 mg in the morning and 30 mg in the evening on day 5, and 30 mg twice daily thereafter
Maintenance dosage: 30 mg twice daily.
Oral Ulcers Associated with Behçet Disease
Oral
Initiate at a dosage of 10 mg daily and titrate as follows (over 5 days in increments of 10 mg daily) to reduce the risk of GI symptoms.
-
10 mg in the morning on day 1
-
10 mg in the morning and 10 mg in the evening on day 2
-
10 mg in the morning and 20 mg in the evening on day 3
-
20 mg in the morning and 20 mg in the evening on day 4
-
20 mg in the morning and 30 mg in the evening on day 5, and 30 mg twice daily thereafter
Maintenance dosage: 30 mg twice daily.
Special Populations
Hepatic Impairment
No dosage adjustment required.
Renal Impairment
Severe renal impairment (Clcr <30 mL/minute): Recommended dosage titration schedule for initiation of therapy in adults is as follows:
- Titration Schedule for Adults
-
10 mg in the morning for 3 days (days 1, 2, and 3)
-
20 mg in the morning for 2 days (days 4 and 5)
-
30 mg once daily thereafter
Severe renal impairment (Clcr <30 mL/minute): Recommended dosage titration schedule for initiation of therapy, based on body weight in pediatric patients, is as follows:
- Titration Schedule for Pediatric Patients ≥50 kg
-
10 mg in the morning for 3 days (days 1, 2, and 3)
-
20 mg in the morning for 2 days (days 4 and 5)
-
30 mg once daily thereafter
- Titration Schedule for Pediatric Patients 20 to <50 kg
-
10 mg in the morning for 3 days (days 1, 2, and 3)
-
20 mg once daily thereafter
Geriatric Patients
Manufacturer makes no specific dosage recommendations.
Cautions for Apremilast
Contraindications
-
Known hypersensitivity to apremilast or any ingredient in the formulation.
Warnings/Precautions
Hypersensitivity
Hypersensitivity reactions including cases of angioedema and anaphylaxis reported. Avoid use in patients with a known hypersensitivity to the drug of any of its excipients.
Discontinue if signs or symptoms of serious hypersensitivity reactions occur and initiate appropriate therapy.
GI Effects
Severe diarrhea, nausea, and vomiting reported.
Monitor patients who are more susceptible to complications, such as patients ≥65 years of age and those taking drugs that can lead to volume depletion or hypotension.
Reduce apremilast dosage or suspend therapy if patients develop severe diarrhea, nausea, or vomiting.
Depression and Suicidality
Depression, depressed mood, and suicidal ideation/behaviors reported.
Carefully weigh risks and benefits prior to using apremilast in patients with a history of depression and/or suicidal thoughts or behavior. Carefully evaluate the risks and benefits of continuing therapy if such effects occur.
Weight Loss
Weight loss of ≥5–10% of body weight reported.
Regularly monitor weight in adults. If unexplained or clinically important weight loss occurs, evaluate weight loss and consider discontinuing apremilast. Monitor growth (height and weight) of pediatric patients closely during therapy. Interruption of therapy may be warranted if patients are not growing or gaining weight as expected.
Interactions
Concomitant use of potent CYP inducers (e.g., carbamazepine, phenobarbital, phenytoin, rifampin) not recommended.
Specific Populations
Pregnancy
Pregnancy registry at 877-311-8972 or [Web] .
Increases in spontaneous abortion and embryofetal death reported in animal reproduction studies.
Lactation
Distributed into milk in mice; not known whether distributed into human milk.
Consider the benefits of breast-feeding and the importance of apremilast to the patient along with potential adverse effects on the breast-fed infant from the drug or underlying maternal condition.
Pediatric Use
Efficacy and safety established in pediatric patients ≥6 year of age weighing ≥20 kg with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Safety and efficacy not established in pediatric patients with psoriatic arthritis or oral ulcers associated with Behçet's disease.
Monitor growth (height and weight) closely in pediatric patients during therapy. May need to interrupt therapy if patients are not growing or gaining weight as expected.
Geriatric Use
No overall differences in safety observed between geriatric and younger adults with psoriatic arthritis.
No overall differences in efficacy and safety observed between geriatric and younger adults with plaque psoriasis.
Monitor patients ≥65 years of age closely for volume depletion or hypotension resulting from treatment-related severe diarrhea, nausea, or vomiting.
Hepatic Impairment
Moderate or severe hepatic impairment (Child-Pugh class B or C) does not alter pharmacokinetics of apremilast. No dosage adjustment necessary.
Renal Impairment
Systemic exposure increased in patients with severe renal impairment (Clcr <30 mL/minute). Reduced dosage recommended.
Common Adverse Effects
Adverse events reported in ≥5% of patients with psoriatic arthritis: Diarrhea, nausea, headache.
Adverse events reported in ≥5% of patients with psoriasis: Diarrhea, nausea, upper respiratory tract infection, headache (including tension headache).
Adverse effects reported in ≥10% of patients with Behçet disease: Diarrhea, nausea, headache, upper respiratory tract infection.
Drug Interactions
Undergoes oxidative metabolism (mediated mainly by CYP3A4, with minor contributions from CYP1A2 and CYP2A6) with subsequent glucuronidation; also undergoes non-CYP-mediated hydrolysis.
Does not inhibit CYP isoenzyme 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4 in vitro; does not induce CYP isoenzyme 1A2, 2B6, 2C9, 2C19, or 3A4 in vitro.
A substrate but not an inhibitor of P-glycoprotein (P-gp) in vitro; neither a substrate nor inhibitor of organic anion transporters (OAT) 1 and 3, organic anion transporting polypeptides (OATP) 1B1 and 1B3, organic cation transporter (OCT) 2, or breast cancer resistance protein (BCRP) in vitro.
Drugs Affecting Hepatic Microsomal Enzymes
Potent CYP inducers: Reduced systemic exposure and possible loss of efficacy of apremilast; concomitant use not recommended.
Specific Drugs
|
Drug |
Interaction |
Comments |
|---|---|---|
|
Anticonvulsants (carbamazepine, phenobarbital, phenytoin) |
Systemic exposure and efficacy of apremilast may be reduced |
Concomitant use not recommended |
|
Estrogens/progestins |
Contraceptive containing ethinyl estradiol and norgestimate: No substantial pharmacokinetic interaction |
|
|
Ketoconazole |
No clinically important effects on apremilast exposure |
|
|
Methotrexate |
No substantial effects on pharmacokinetics of either drug |
|
|
Rifampin |
Reduced apremilast AUC and peak plasma concentration; possible loss of apremilast efficacy |
Concomitant use not recommended |
Apremilast Pharmacokinetics
Absorption
Bioavailability
Absolute bioavailability of about 73%. Peak plasma concentrations achieved about 2.5 hours after oral administration.
Food
Food does not alter extent of absorption.
Special Populations
Moderate or severe hepatic impairment does not alter apremilast pharmacokinetics.
In patients with severe renal impairment, AUC and peak plasma concentrations increased by approximately 88 and 42%, respectively. Mild to moderate renal impairment did not affect apremilast pharmacokinetics.
In healthy geriatric individuals (65–85 years of age), AUC and peak plasma concentrations increased by about 13 and 6%, respectively, compared with younger adults (18–55 years of age).
In healthy women, extent of exposure and peak plasma concentrations increased by about 31 and 8%, respectively, compared with men.
Apremilast exposure is similar among Hispanic white, non-Hispanic white, and African Americans; pharmacokinetics in healthy Chinese and Japanese men similar to that in healthy white men.
Distribution
Extent
Not known whether apremilast distributes into milk.
Plasma Protein Binding
Approximately 68%.
Elimination
Metabolism
Undergoes oxidative metabolism (mediated mainly by CYP3A4, with minor contributions from CYP1A2 and CYP2A6) with subsequent glucuronidation; also undergoes non-CYP-mediated hydrolysis.
Elimination Route
Following oral administration of radiolabeled apremilast, radioactivity recovered in urine (58%) and feces (39%), with about 3 or 7% of dose recovered as unchanged drug in urine or feces, respectively.
Half-life
Approximately 6–9 hours.
Stability
Storage
Oral
Tablets
Store at <30°C.
Actions
-
Selectively inhibits PDE4, the dominant phosphodiesterase expressed in immune cells and a major enzyme involved in metabolism of cyclic adenosine-3',5'-monophosphate (cAMP).
-
Selective inhibition of PDE4 results in accumulation of intracellular cAMP, a modulator of inflammatory responses, which can down-regulate inflammatory responses by increasing expression of anti-inflammatory mediators and partially inhibiting expression of inflammatory cytokines that are thought to play a role in the pathogenesis of psoriasis and active psoriatic arthritis.
Advice to Patients
-
Inform patients that apremilast may be taken without regard to meals. Advise patients to not crush, split, or chew apremilast tablets.
-
Advise patients of the potential for serious allergic reactions following administration and to contact a clinician if symptoms of an allergic reaction occur.
-
Advise patients to contact a clinician if they experience severe diarrhea, nausea, or vomiting.
-
Risk of emergence or worsening of depression. Instruct patients and their caregivers or families to be alert for the emergence or worsening of depression, suicidal thoughts, or other mood changes and to contact a clinician if such changes occur.
-
Risk of weight loss. Stress importance of regularly monitoring for weight loss; advise patients to inform clinician if substantial or unexplained weight loss occurs.
-
Risk of interactions with potent CYP inducers (e.g., carbamazepine, phenobarbital, phenytoin, rifampin). Advise patients to inform their clinician of existing or contemplated concomitant therapy, including prescription and OTC drugs and dietary and herbal supplements, as well as any concomitant illnesses.
-
Advise women to inform their clinician if they are or plan to become pregnant or plan to breast-feed.
-
Advise patients of other important precautionary information.
Additional Information
The American Society of Health-System Pharmacists, Inc. represents that the information provided in the accompanying monograph was formulated with a reasonable standard of care, and in conformity with professional standards in the field. Readers are advised that decisions regarding use of drugs are complex medical decisions requiring the independent, informed decision of an appropriate health care professional, and that the information contained in the monograph is provided for informational purposes only. The manufacturer’s labeling should be consulted for more detailed information. The American Society of Health-System Pharmacists, Inc. does not endorse or recommend the use of any drug. The information contained in the monograph is not a substitute for medical care.
Preparations
Excipients in commercially available drug preparations may have clinically important effects in some individuals; consult specific product labeling for details.
Please refer to the ASHP Drug Shortages Resource Center for information on shortages of one or more of these preparations.
* available from one or more manufacturer, distributor, and/or repackager by generic (nonproprietary) name
|
Routes |
Dosage Forms |
Strengths |
Brand Names |
Manufacturer |
|---|---|---|---|---|
|
Oral |
Kit |
4 tablets, film-coated, apremilast 10 mg 4 tablets, film-coated, apremilast 20 mg 47 tablets, film-coated, apremilast 30 mg |
Otezla 28-day Treatment Initiation Pack (configuration for 30 mg twice daily dosage) |
Amgen |
|
4 tablets, film-coated, apremilast 10 mg 51 tablets, film-coated, apremilast 20 mg |
Otezla 28-Day Treatment Initiation Pack (configuration for 20 mg twice daily dosage) |
Amgen |
||
|
Tablets, film-coated |
10 mg* |
Apremilast Tablets |
||
|
Otezla |
||||
|
20 mg* |
Apremilast Tablets |
|||
|
Otezla |
||||
|
30 mg* |
Apremilast Tablets |
|||
|
Otezla |
Amgen |
AHFS DI Essentials™. © Copyright 2025, Selected Revisions April 10, 2025. American Society of Health-System Pharmacists, Inc., 4500 East-West Highway, Suite 900, Bethesda, Maryland 20814.
Reload page with references included
Related/similar drugs
Frequently asked questions
- Sotyktu vs Otezla: How do they compare?
- How long does it take for Otezla to work?
- Who makes Otezla and why is it so expensive?
- How does Otezla work and what's its MOA?
- What are the new drugs for plaque psoriasis?
- Is Otezla (apremilast) an immunosuppressant?
More about apremilast
- Check interactions
- Compare alternatives
- Reviews (478)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: antirheumatics
- Breastfeeding
- En español