Skytrofa
Generic name: lonapegsomatropin
Dosage form: injection (3 mg, 3.6 mg, 4.3 mg, 5.2 mg, 6.3 mg, 7.6 mg, 9.1 mg, 11 mg, 13.3 mg)
Drug class: Growth hormones
What is Skytrofa?
Skytrofa is a human growth hormone injection used to increase growth in children with pediatric growth hormone deficiency. Skytrofa injections are used in children 1 year and older who have growth problems due to a lack of natural growth hormone. Skytrofa works by acting like a natural human growth hormone binding to growth hormone (GH) receptors and is important not only for height but also for a child’s overall endocrine health and development.
Skytrofa injection is given once a week under the skin of the abdomen, thigh, or buttocks using the Skytrofa autoinjector. It is a slow-release injection which means it only needs to be given once a week instead of daily. It may be stored at room temperature and is preservative-free. Skytrofa contains a slow-release form of somatropin called lonapegsomatropin.
Pediatric growth hormone deficiency occurs when the pituitary gland in the child's body does not make enough growth hormone. Growth hormone deficiency usually results in a child having slow growth and short stature, and it may also affect how the body manages fats, proteins, and carbohydrates.
Skytrofa FDA approval
Skytrofa FDA approval is for the treatment of children one year and older who weigh at least 11.5 kg and have growth failure due to inadequate secretion of endogenous growth hormone. FDA approval was granted to Ascendis Pharma on August 25, 2021.
FDA approval of Skytrofa was based on results of the phase 3 heiGHt Trial (NCT02781727) that compared once-weekly Skytrofa to once-daily somatropin (Genotropin) over 52 weeks.
Skytrofa patients had a:
- higher annualized height velocity (AHV) 11.2 cm/year compared to once daily somatropin 10.3 cm/year
- Skytrofa and somatropin (Genotropin) had similar safety and tolerability.
Skytrofa side effects
Common Skytrofa side effects
Common Skytrofa side effects may include
- cold symptoms (15%)
- fever (15%),
- cough (11%)
- nausea and vomiting (11%)
- bleeding problems such as nosebleeds, pinpoint purple or red spots under your skin (7%)
- diarrhea (6%)
- stomach pain (6%)
- joint pain (6%)
These are the common side effects that affected 5% or more of Skytrofa patients in a clinical trial (NCT02781727).
Serious Skytrofa side effects
Get emergency medical help if you have signs of an allergic reaction, such as hives, difficulty breathing, or swelling of your face, lips, tongue, or throat.
Skytrofa may cause other serious side effects. Call your doctor at once if you have:
- pain in your knees or hips, walking with a limp;
- numbness or tingling in your wrist, hand, or fingers;
- severe swelling or puffiness in your hands and feet;
- changes in behavior;
- vision problems, unusual headaches;
- changes in the shape or size of a mole;
- pain or swelling in your joints;
- Pancreatitis - severe pain in your upper stomach spreading to your back, nausea and vomiting;
- high blood sugar - increased thirst, increased urination, dry mouth, fruity breath odor;
- increased pressure inside the skull--severe headaches, ringing in your ears, dizziness, nausea, vision problems, pain behind your eyes; or
- signs of an adrenal gland problem--extreme weakness, severe dizziness, weight loss, changes in skin color, feeling very weak or tired.
This is not a complete list of side effects, and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Related/similar drugs
Humatrope
Humatrope injection (somatropin) is a human growth hormone used to treat growth failure in children ...
Ngenla
Ngenla is a long-acting human growth hormone used to treat children aged 3 and older who are not ...
Sogroya
Sogroya information from Drugs.com, includes Sogroya side effects, interactions and indications.
Genotropin
Genotropin is a human growth hormone used to treat growth failure in children and adults who lack ...
Norditropin
Norditropin is used to treat growth hormone deficiency (GHD) in adults and children and other ...
Omnitrope
Omnitrope (somatropin) is a form of human growth hormone used to treat adults and children with ...
Norditropin FlexPro
Norditropin FlexPro is used for adult human growth hormone deficiency, idiopathic short stature ...
Nutropin
Nutropin is used for adult human growth hormone deficiency, growth retardation, chronic renal ...
Nutropin AQ
Nutropin AQ (somatropin) is an injectable preparation that may be used to treat children with ...
Warnings
Call your doctor at once if you have severe pain in your upper stomach spreading to your back, increased thirst, increased urination, severe headaches, ringing in your ears, dizziness, nausea, vision problems, weight loss, changes in skin color, feeling very weak or tired.
Before taking this medicine
You should not use Skytrofa if you are allergic to it or if you have:
- a serious illness due to lung failure or complications from recent surgery, injury, or medical trauma;
- closed epiphyses;
- active cancer;
- eye problems caused by diabetes (diabetic retinopathy); or
- Prader-Willi syndrome, and you are overweight or have severe breathing problems (including sleep apnea).
Tell your doctor if you have ever had:
- cancer (especially during childhood);
- diabetes;
- a head injury or brain tumor;
- childhood brain cancer and radiation treatment;
- a pituitary gland disorder;
- underactive thyroid;
- abnormal curvature of the spine (scoliosis); or
- breathing problems, sleep apnea (breathing stops during sleep).
Tell your doctor if you are pregnant or breastfeeding.
How is Skytrofa given?
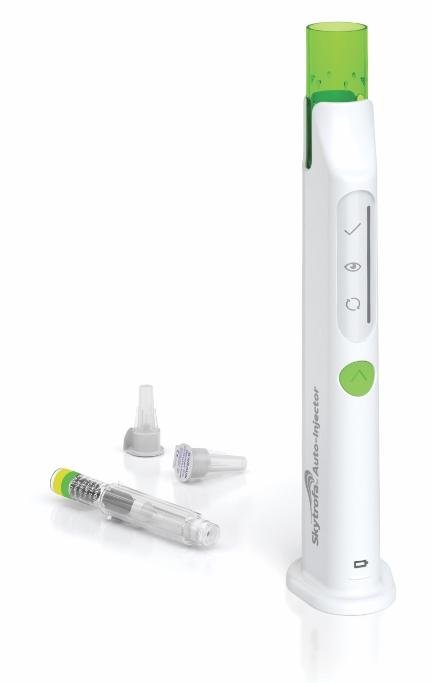
Skytrofa is injected under the skin once a week using the Skytrofa Auto-Injector. It can be injected under the skin of the abdomen, thigh, or buttocks. Rotate injection sites between and within regions to reduce the risk of lipoatrophy. Do not inject into the same place two times in a row.
The Skytrofa Auto-Injector is an electronic, reusable device, that automates parts of the procedure for injecting Skytrofa so it can be used at home by the the patient or caregiver.
A healthcare provider can teach you how to properly use the Skytrofa auto-injector by yourself or for your child.
If the injection has been kept refrigerated, remove it from the fridge and keep it at room temperature for 15 minutes before use.
Prepare an injection only when you are ready to give it.
The Skytrofa Auto-Injector must be used with the Skytrofa cartridge. The cartridge has 2 chambers, 1 filled with powder and 1 filled with water. The auto-injector automatically mixes the powder and the water during preparation, making it ready for injection.
The mixed solution should be clear and colorless to opalescent and may occasionally contain air bubbles. You should NOT inject if the solution is cloudy or contains particulate matter.
When the injection needle is inserted into the skin, the device automatically delivers the drug product. The built-in electronics and software assist the user during the entire preparation and injection sequence and provide confirmation that the full dose has been delivered.
Use cartridges within 4 hours of reconstitution. When stored at room temperature up to 86°F (30°C), discard reconstituted cartridges after 4 hours.
Do not reuse a needle or syringe. Place them in a puncture-proof "sharps" container and dispose of it following state or local laws. Keep out of the reach of children and pets.
Never share an injection pen, cartridge, or syringe even if you changed the needle. Sharing these devices can pass infections from person to person.
You may need to have frequent medical tests.
Follow all directions on your prescription label and read all medication guides or instruction sheets. Use the medicine exactly as directed.
Read and carefully follow any Instructions for Use provided with your medicine. If you don't understand how to use an injection, ask your doctor or pharmacist.
Skytrofa dosage information
The recommended Skytrofa dose for treatment-naïve patients and patients switching from daily somatropin therapy is 0.24 mg/kg body weight, given once weekly.
Maintenance Skytrofa dose: Individualize and titrate the dosage based on response.
Changing from daily somatropin therapy to once-weekly Skytrofa: wait at least 8 hours between the final dose of daily somatropin and the first dose of once-weekly Skytrofa.
Comments: If patients experience failure to increase height velocity, particularly during the first year of treatment, assess compliance and evaluate other causes of poor growth, such as hypothyroidism, undernutrition, advanced bone age, and antibodies to recombinant human growth hormone.
Skytrofa should be discontinued once epiphyseal fusion has occurred.
What happens if I miss a dose?
Use the medicine as soon as you can, but skip the missed dose if you are more than 2 days late for the dose. Do not use two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
Overdose can cause tremors or shaking, cold sweats, increased hunger, headache, drowsiness, weakness, dizziness, fast heartbeat, and nausea. Long-term overdose may cause excessive growth.
What should I avoid while using Skytrofa?
Follow your doctor's instructions about any restrictions on food, beverages, or activity.
What other drugs will affect Skytrofa?
Sometimes it is not safe to use certain medicines at the same time. Some drugs can affect your blood levels of other drugs you use, which may increase side effects or make the medicines less effective.
Tell your doctor about all your other medicines, especially:
- birth control pills or hormone replacement therapy;
- insulin or oral diabetes medicine; or
- a steroid (prednisone, dexamethasone, methylprednisolone, and others).
This list is not complete. Other drugs may affect Skytrofa; including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here. For more information on interactions with Skytrofa, click the link below.
Skytrofa Package Insert
HCPs and patients often use the Skytrofa Package Insert (PI) for more detailed information about this medicine. The Package Insert (prescribing information) contains more comprehensive information on Indications and Usage, Dosage and Administration, Clinical Pharmacology, Clinical Studies, Drug Interaction, and more. Discuss any medical questions you have with your HCP (health care professional). This is not all the information you need to know about this medicine for safe and effective use, and it does not take the place of talking to your doctor about your treatment.
The Package Insert is sometimes called Skytrofa Prescribing Information (PI) or FDA label.
Storage
Patients storage
Fridge storage
- Refrigerate cartridges at 36°F to 46°F (2°C to 8°C). Do not freeze
- Keep cartridges in the outer carton to protect them from light.
- Skytrofa may be used until the expiration date when stored in a refrigerator.
Room temperature storage
- Outer cartons containing blistered cartridges may be stored at room temperature [up to 86°F (30°C)] for up to 6 months and can be returned to refrigeration within the 6 months. Write the date first removed from the refrigerator in the space provided on the outer carton.
- Do not use Skytrofa beyond the expiration date or 6 months after the date it was first removed from refrigeration (whichever is earlier).
Pharmacy Storage
- Cartridges should be stored refrigerated at 36°F to 46°F (2°C to 8°C) in the outer carton to protect them from light until the expiration date.
- Do not freeze.
Company
Skytrofa Ascendis Pharma Endocrinology Division A/S, Tuborg Boulevard 12 Hellerup Denmark DK-2900
Skytrofa Biosimilars
Biosimilar and interchangeable products are biological products that are highly similar to and have no clinically meaningful differences from the reference product.
Reference products
These are biological products that have already been approved by the FDA, against which biosimilar products are compared. There is 1 for Skytrofa.
Skytrofa (lonapegsomatropin-tcgd) - Ascendis Pharma Endocrinology Division A/S
| Formulation type | Strength |
|---|---|
| Single-Dose Cartridge | 11 mg |
| Single-Dose Cartridge | 13.3 mg |
| Single-Dose Cartridge | 3.6 mg |
| Single-Dose Cartridge | 3 mg |
| Single-Dose Cartridge | 4.3 mg |
| Single-Dose Cartridge | 5.2 mg |
| Single-Dose Cartridge | 6.3 mg |
| Single-Dose Cartridge | 7.6 mg |
| Single-Dose Cartridge | 9.1 mg |
References
More about Skytrofa (lonapegsomatropin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: growth hormones
- Breastfeeding
- En español
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.