Panzyga: Package Insert / Prescribing Info
Package insert / product label
Generic name: immune globulin intravenous (human)
Dosage form: injection
Drug class: Immune globulins
J Code (medical billing code): J1599 (500 mg, injection)
Medically reviewed by Drugs.com. Last updated on Apr 14, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
PANZYGA, (immune globulin intravenous, human - ifas)
10% Liquid Preparation
Initial U.S. Approval: 2018
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
See full prescribing information for complete boxed warning
- Thrombosis may occur with immune globulin intravenous (IGIV) products, including PANZYGA. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
- Renal dysfunction, acute renal failure, osmotic nephropathy, and death may occur with the administration of IGIV products in predisposed patients. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. PANZYGA does not contain sucrose.
-For patients at risk of thrombosis, renal dysfunction, or renal failure, administer PANZYGA at the minimum infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Recent Major Changes
Indications and Usage for Panzyga
PANZYGA is an immune globulin intravenous (human) - ifas 10% liquid preparation indicated for the treatment of:
• Primary humoral immunodeficiency (PI) in patients 2 years of age and older ( 1.1 ).
• Chronic immune thrombocytopenia (ITP) in adults ( 1.2 ).
• Chronic inflammatory demyelinating polyneuropathy (CIDP) in adults (1.3)
Panzyga Dosage and Administration
For intravenous use only ( 2 ).
| Indication | Dose | Initial Infusion Rate | Maximum Infusion Rate (as tolerated) |
| PI | 300-600 mg/kg (3-6 mL/kg)every 3-4 weeks | 1 mg/kg/min (0.01 mL/kg/min) | 14 mg/kg/min (0.14 mL/kg/min) |
| Chronic ITP in adults | 2 g/kg (20 mL/kg), divided into 2 daily doses of 1 g/kg (10 mL/kg) given on 2 consecutive days | 1 mg/kg/min (0.01 mL/kg/min) | 8 mg/kg/min (0.08 mL/kg/min) |
| CIDP in adults | Loading dose: 2 g/kg (20 mL/kg), divided into 2 daily doses of 1 g/kg (10 mL/kg) given on 2 consecutive days Maintenance dose: 1-2 g/kg (10-20 mL/kg) every 3 weeks divided in 2 doses given over 2 consecutive days | 1 mg/kg/min (0.01 mL/kg/min) | 12 mg/kg/min (0.12 mL/kg/min) |
• Ensure that patients with pre-existing renal insufficiency are not volume depleted; discontinue PANZYGA if renal function deteriorates ( 2 , 5.2 ).
• For patients at risk of renal dysfunction or thrombotic events, administer PANZYGA at the minimum dose and infusion rate practicable ( 5.2 , 5.4 ).
Contraindications
Warnings and Precautions
• IgA-deficient patients with antibodies against IgA are at greater risk of developing severe hypersensitivity and anaphylactic reactions to PANZYGA. Epinephrine should be available immediately to treat any severe acute hypersensitivity reactions ( 5.1 ).
• Monitor renal function, including blood urea nitrogen and serum creatinine, and urine output in patients at risk of developing acute renal failure ( 5.2 ).
• Hyperproteinemia, increased serum osmolarity and hyponatremia may occur in patients receiving PANZYGA ( 5.3 ).
• Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to PANZYGA treatments. Risk factors for hemolysis include high doses and non-O-blood group. Closely monitor patients for hemolysis and hemolytic anemia ( 5.6 ).
• Aseptic Meningitis Syndrome may occur in patients receiving PANZYGA, especially with high doses or rapid infusion ( 5.5 ).
• Monitor patients for pulmonary adverse reactions [transfusion-related acute lung injury (TRALI)] ( 5.7 ).
• PANZYGA is made from human plasma and may contain infectious agents, e.g., viruses, variant Creutzfeldt-Jakob disease [vCJD] agent and, theoretically, the Creutzfeldt-Jakob disease agent ( 5.10 ).
Adverse Reactions/Side Effects
• PI - The most common adverse reactions reported in greater than 5% of subjects during a clinical trial were: headache, nausea, fever, fatigue, and abdominal pain ( 6 ).
• Chronic ITP in adults - The most common adverse reactions reported in greater than 5% of subjects during a clinical trial were: headache, fever, nausea, vomiting, dizziness, and anemia ( 6 ).
• CIDP in adults – The most common adverse reactions reported in greater than 5% of subjects during a clinical trial were: headache, fever, dermatitis, blood pressure increased ( 6 ).
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Use In Specific Populations
Geriatric Use: In patients over age 65 years and in any patient at risk of developing renal insufficiency, infuse PANZYGA at the minimum infusion rate practicable and do not exceed the recommended dose PANZYGA ( 8.5 ).
_______________________________________________________________________________________________________________________________________
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2025
Full Prescribing Information
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
Thrombosis may occur with immune globulin intravenous (IGIV) products, including PANZYGA. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. (See WARNING and PRECAUTIONS [ 5.4 ], PATIENT COUNSELING INFORMATION [ 17 ])
Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur in predisposed patients who receive IGIV products, including PANZYGA. Patients predisposed to renal dysfunction include those with a degree of pre-existing renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV product containing sucrose. PANZYGA does not contain sucrose.
For patients at risk of thrombosis, renal dysfunction or acute renal failure, administer PANZYGA at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. (See DOSAGE and ADMINISTRATION [ 2.3 ], WARNINGS and PRECAUTIONS [ 5.2 , 5.4 ])
1. Indications and Usage for Panzyga
1.1 Primary Humoral Immunodeficiency Diseases (PI)
PANZYGA is indicated for treatment of primary humoral immunodeficiency (PI) in patients 2 years of age and older. This includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
2. Panzyga Dosage and Administration
For intravenous use only.
2.1 Dose
| Indication | Dose | Initial Infusion Rate (first 30 min) | Maximum Infusion Rate (as tolerated) |
|
Treatment of Primary Humoral Immunodeficiency (PI)*
| 300 to 600 mg/kg body weight (3-6 mL/kg) administered every 3 to 4 weeks |
1 mg/kg/min (0.01 mL/kg/min) |
14 mg/kg/min (0.14 mL/kg/min) |
|
Treatment of Chronic Immune Thrombocytopenia (ITP)
| 2 g/kg (20 mL/kg), divided into 2 daily doses of 1 g/kg (10 mL/kg) given on 2 consecutive days |
1 mg/kg/min (0.01 mL/kg/min) |
8 mg/kg/min (0.08 mL/kg/min) |
| Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) |
Loading Dose: 2 g/kg (20 mL/kg), divided into 2 daily doses of 1 g/kg (10 mL/kg) given on 2 consecutive days Maintenance dose: 1-2 g/kg (10-20 mL/kg) every 3 weeks divided in 2 daily doses given over 2 consecutive days | 1 mg/kg/min (0.01 mL/kg/min) | 12 mg/kg/min (0.12 mL/kg/min) |
*Significant differences in the half-life of IgG among patients with PI may necessitate the dose and frequency of immunoglobulin therapy to vary from patient to patient. Determine the proper dose and frequency by monitoring the clinical response. Adjust dose over time to achieve the desired trough levels of IgG and clinical responses.
The initial infusion rate should be maintained for 30 min. Following the initial infusion, and if tolerated, the infusion rate may be gradually increased every 15-30 minutes, as tolerated to the maximum infusion rate shown in the table above for each indication.
Measles Exposure.
If a patient with primary humoral immunodeficiency has been exposed to measles, it may be prudent to administer an extra dose of IGIV as soon as possible and within 6 days of exposure. A dose of 400 mg/kg should provide a serum level > 240 mIU/mL of measles antibodies for at least two weeks.
If a patient with primary humoral immunodeficiency is at risk of future measles exposure and receives a dose of less than 530 mg/kg every 3-4 weeks, the dose should be increased to at least 530 mg/kg. This should provide a serum level of 240 mIU/mL of measles antibodies for at least 22 days after infusion.
2.2 Administration
- Inspect parenteral products visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use PANZYGA if it is turbid and/or if discoloration is observed. Using a needle, no larger than a 16-gauge needle, insert the needle only once within the stopper area (delineated by the raised ring for penetration). Penetrate the stopper perpendicularly to its plane and within the ring.
- PANZYGA bottles may be pooled under aseptic conditions into sterile infusion bags. Infuse within 8 hours after pooling.
- Administer at room or body temperature only by the intravenous route
- PANZYGA is not supplied with an infusion set. If a filtered infusion set is used (not mandatory), choose a filter size of 0.2-200 microns.
- Do not administer PANZYGA simultaneously with another intravenous preparation in the same infusion set, including immune globulin products from another manufacturer.
- After administration, the infusion line may be flushed with either normal saline or 5% dextrose in water.
- Monitor the patient carefully throughout the infusion. Certain adverse drug reactions are related to the rate of infusion, and will disappear promptly after slowing or stopping the infusion. In such cases, after symptoms subside, resume the infusion at a lower rate. Ensure that patients with pre-existing renal insufficiency are not volume depleted. For patients at risk of renal dysfunction or thromboembolic events, administer PANZYGA at the minimum infusion rate practicable [ see Warnings and Precautions (5.2, 5.4)] . Do not exceed 3.3 mg/kg/min (0.033 mL/kg/min). Discontinue if renal function deteriorates.
3. Dosage Forms and Strengths
Solution containing 10% IgG (100 mg/mL) (See How Supplied/Storage and Handling ( 16 )).
4. Contraindications
- PANZYGA is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin.
- PANZYGA is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity .
5. Warnings and Precautions
5.1 Hypersensitivity
Severe hypersensitivity reactions may occur (See Contraindications ( 4 )) . [ 1 ] In case of hypersensitivity, discontinue PANZYGA infusion immediately and institute appropriate treatment. Have epinephrine available for immediate treatment of severe acute hypersensitivity reactions.
PANZYGA contains trace amounts of IgA (average 100 µg/mL in a 10% solution). IgA-deficient patients with antibodies against IgA are at greater risk of developing severe hypersensitivity and anaphylactoid reactions when administered PANZYGA (See Contraindications ( 4 )) .
5.2 Renal Failure
Renal dysfunction, acute renal failure, osmotic nephropathy, and death may occur upon use of PANZYGA in predisposed patients.
Ensure that patients are not volume depleted prior to the initiation of the infusion of PANZYGA.
For patients at risk of renal dysfunction because of pre-existing renal insufficiency or predisposition to acute renal failure (such as individuals with diabetes mellitus, age greater than 65 years, volume depletion, sepsis, paraproteinemia, or those receiving known nephrotoxic drugs), administer PANZYGA at the minimum infusion rate practicable (See Boxed Warning, and Dosage and Administration ( 2 )).
Periodic monitoring of renal function tests and urine output is particularly important in patients judged to be at risk of developing acute renal failure. Assess renal function, including a measurement of blood urea nitrogen (BUN)/serum creatinine, prior to the initial infusion of PANZYGA and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation of the product.
5.3 Hyperproteinemia, Increased Serum Viscosity and Hyponatremia
Hyperproteinemia, increased serum viscosity and hyponatremia may occur in patients receiving PANZYGA therapy. It is clinically critical to distinguish true hyponatremia from pseudohyponatremia related to hyperproteinemia with concomitant decreased calculated serum osmolality or elevated osmolar gap, because treatment aimed at decreasing serum free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity and a higher risk of thromboembolic events.[ 2 ]
5.4 Thrombotic Events
Thrombosis may occur following treatment with immune globulin products, including PANZYGA. Risk factors include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia / markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients at risk of thrombotic events, administer PANZYGA at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.[ 3-5 ] (See BOXED WARNING, DOSAGE and ADMINSTRATION [ 2 ], PATIENT COUNSELING INFORMATION [ 17 ])
5.5 Aseptic Meningitis Syndrome
Aseptic meningitis syndrome (AMS) may occur with PANZYGA treatment. Discontinuation of treatment has resulted in remission of AMS within several days without sequelae. The syndrome usually begins within several hours to two days following infusion with PANZYGA. It is characterized by symptoms and signs including severe headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea, and vomiting. Cerebrospinal fluid (CSF) studies frequently reveal pleocytosis up to several thousand cells per cubic millimeter, predominantly from the granulocytic series, and elevated protein levels up to several hundred mg/dl, but negative culture results. Conduct a thorough neurological examination in patients exhibiting such symptoms and signs, including CSF studies, to rule out other causes of meningitis. Patients with a history of migraine may be more susceptible.[ 6 ]
AMS may occur more frequently following high doses (≥ 2 g/kg) and/or rapid infusion of IGIV.
5.6 Hemolysis
PANZYGA may contain blood group antibodies that can act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin test (DAT) (Coombs’ test) result and hemolysis. Delayed hemolytic anemia can develop subsequent to IGIV therapy due to enhanced RBC sequestration and acute hemolysis, consistent with intravascular hemolysis, has been reported. Cases of severe hemolysis-related renal dysfunction/failure or disseminated intravascular coagulation have occurred following infusion of IGIV.
The following risk factors may be associated with the development of hemolysis following IGIV administration: high doses (e.g., 2 g/kg or more), given either as a single administration or divided over several days, and non-O blood group.[ 7 ] Other individual patient factors, such as an underlying inflammatory state (as may be reflected by, for example, elevated C-reactive protein or erythrocyte sedimentation rate), have been hypothesized to increase the risk of hemolysis following administration of IGIV [ 8 ], but their role is uncertain. Hemolysis has been reported following administration of IGIV for a variety of indications, including ITP.
Closely monitor patients for clinical signs and symptoms of hemolysis, particularly patients with risk factors noted above. Consider appropriate laboratory testing in higher risk patients, including measurement of hemoglobin or hematocrit prior to infusion and within approximately 36 to 96 hours post-infusion. If clinical signs and symptoms of hemolysis or a significant drop in hemoglobin or hematocrit have been observed, perform confirmatory laboratory testing, including direct antiglobulin test. If transfusion is indicated for patients who develop hemolysis with clinically compromising anemia after receiving IGIV, perform adequate cross-matching to avoid exacerbating on-going hemolysis.
5.7 Transfusion-Related Acute Lung Injury (TRALI)
Noncardiogenic pulmonary edema [Transfusion-Related Acute Lung Injury (TRALI)] may occur in patients administered IGIV.[ 9 ] TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Signs and symptoms typically appear within 1 to 6 hours after transfusion. Patients with TRALI may be managed using oxygen therapy with adequate ventilatory support.
Monitor recipients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-HLA and anti-neutrophil antibodies in both the product and patient’s serum.
5.8 Hypertension
Elevations of systolic blood pressure to 180 mm Hg or more and/or of diastolic blood pressure to more than 120 mm Hg (hypertensive urgency) can be observed during and/or shortly following infusion of IGIV. Such elevations are reported more often among patients with a history of hypertension. Check patients for a history of hypertension and current antihypertensive medication use. Monitor blood pressure prior to, during, and following PANZYGA infusion.
5.9 Volume Overload
Carefully consider the relative risks and benefits before prescribing the high dose regimen (for chronic ITP) in patients at increased risk of volume overload.
5.10 Transmission of Infectious Agents
Because PANZYGA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses and theoretically, the variant Creutzfeldt-Jakob disease and Creutzfeldt-Jakob disease (CJD) agent. The risk of infectious agent transmission has been reduced by screening plasma donors and by including virus inactivation/removal steps in the manufacturing process of PANZYGA. Report all infections thought by a physician or other healthcare provider to have been possibly transmitted by this product to Pfizer Inc. at 1-800-438-1985. Discuss the risks and benefits of PANZYGA with the patient before prescribing or administering this product.
5.11 Monitoring Laboratory Tests
- After infusion of immunoglobulin, the transitory rise of the passively transferred antibodies in the patient’s blood may yield positive serological testing results, with the potential for misleading interpretation.
- Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs’) test. Clinically assess patients with known renal dysfunction, diabetes mellitus, age greater than 65 years, volume depletion, sepsis, paraproteinemia, or those receiving nephrotic agents, and monitor as appropriate (BUN; serum creatinine, urine output) during therapy with PANZYGA.
- Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with polycythemia, cryoglobulins, fasting chylomicronemia/markedly high triglycerides, or monoclonal gammopathies.
- Consider measuring hemoglobin or hematocrit at baseline and approximately 36 to 96 hours post-infusion in patients at higher risk of hemolysis. If signs and/or symptoms of hemolysis are present after an infusion of PANZYGA, perform appropriate laboratory testing for confirmation.
- If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and patient’s serum.
6. Adverse Reactions/Side Effects
PI: The most common adverse reactions observed at a rate of more than 5% in subjects in clinical trials were: headache, abdominal pain, fever, nausea, and fatigue.
Chronic ITP in adults: The most common adverse reactions observed at a rate of more than 5% in subjects in clinical trials were: headache, fever, nausea, vomiting, dizziness, and anemia.
CIDP in adults: The most common adverse reactions observed at a rate of more than 5% in subjects in clinical trials were: headache, fever, dermatitis and blood pressure increased.
The most serious adverse reaction observed with PANZYGA treatment during clinical trials was aseptic meningitis in one subject.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in clinical practice.
Treatment of Primary Humoral Immunodeficiency (PI)
In a prospective, open-label, single-arm, multicenter study in 51 children and adults with PI, subjects received PANZYGA at a dose between 200 to 800 mg/kg body weight every 3 or 4 weeks. Subjects participated in the study for a mean of 360 days. Infusions were initiated at a rate of 1 mg/kg/minute for the first 30 minutes, and, if tolerated, could be advanced to a maximum tolerated rate not exceeding 8 mg/kg/minute. The mean age of subjects was 26.8 years (range: 2 to 65 years).
This study was followed by an extension study that evaluated the safety of PANZYGA administered at higher infusion rates in 21 subjects that successfully had completed the first study. Nineteen of the 21 enrolled patients received PANZYGA up to the maximum allowed infusion rate of 14 mg/kg/minute. In the extension study, medication-related adverse events were reported in 4 of 21 subjects (19%). Adverse reactions occurring in more than 5% of subjects were nausea (9.5%) and headache (9.5%).
In the study in PI, infusion-related adverse events (during or within 72 hours after the end of infusion) were reported in 16 patients (76%) enrolled in the 3-weeks treatment schedule and in 22 patients (73%) in the 4-weeks treatment schedule. Overall, 38 infusions (5%) had at least one adverse event considered related to study medication: 5 infusions (3%) in children, 4 infusions in adolescents (2%), and 29 infusions (8%) in adults. Study medication-related (possible or probable) infusion-related adverse reactions were associated with 35 infusions (5%) (overall); study medication-related headache was noted in 21 infusions (3%).
Table 1: Adverse Reactions* Occurring in more than 5% of Subjects with PI
| Adverse Reaction | No. of Subjects with Adverse Reaction(percentage of subjects) |
| Headache | 11 (22%) |
| Abdominal pain (upper) | 7 (14%) |
| Fever | 7 (14%) |
| Nausea | 5 (10%) |
| Sinusitis | 4 (8%) |
| Fatigue | 3 (6%) |
| Bronchitis | 3 (6%) |
* Any infusional and any study medication related adverse events.
Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
In a prospective, open-label, single-arm, multicenter study, 40 adult subjects with chronic ITP received PANZYGA at a dose of 2 g/kg, administered daily as 1 g/kg intravenous infusions on 2 consecutive days. 3/40 subjects did not receive a second infusion of PANZYGA due to infusion reactions, including chills, headache, fever and nausea. All subjects except 1 received at least 1 infusion with the highest rate of 8 mg/kg/minute. Pre-medication to alleviate potential adverse drug reactions was not allowed in the study.
There were 67 treatment emergent adverse events (TEAEs) reported in 24 (60%) subjects that were related to administration of PANZYGA. 55 of these adverse events ( 82%) were infusional adverse events that occurred within 72 hours after start of the infusion. Seven of these adverse events in 2 subjects were severe. These included headache, nausea, vomiting and chills.
When analyzed by infusion, infusion-related adverse events were reported in 33 of the 77 infusions (43%).
Table 2: Adverse Reactions* Occurring in more than 5% of Subjects with Chronic ITP in Adults
| Adverse Reaction |
No. of Subjects with Adverse Reaction(percentage of subjects)
|
| Headache | 20 (50%) |
| Fever | 9 (23%) |
| Nausea | 7 (18%) |
| Vomiting | 4 (10%) |
| Dizziness | 4 (10%) |
| Anemia | 4(10%) |
* Any infusional and any study medication related adverse events.
One out of 40 subjects with ITP treated with PANZYGA developed aseptic meningitis on Day 2 of the infusion. This subject was managed with antibiotics and supportive care with recovery.
Baseline direct Coomb’s test was performed in 39/40 subjects that were treated with PANZYGA. 10/39 (26%) subjects subsequently developed positive Coomb’s test. One subject was not tested at baseline but had positive results on all 3 subsequent visits. Four of these subjects (10%) developed hemolytic anemia after receiving PANZYGA. These resolved spontaneously without any intervention.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy in Adults
In a prospective, double-blind, randomized, multicenter study, 142 adult subjects with CIDP aged between 18 and 83 years were enrolled and randomized 1:2:1 to receive first a loading dose of 2 g/kg and then 0.5 g/kg, 1.0 g/kg or 2.0 g/kg PANZYGA for 7 maintenance infusions at 3-week intervals during the 24-week Dose-evaluation Phase (mean doses administered - including loading and rescue doses - were 0.91, 1.24 and 1.97 g/kg for 0.5, 1 and 2g/kg group, respectively).
All 142 subjects in this study received at least 1 dose of PANZYGA. The median maximum infusion rate was 0.12 mL/kg/min throughout the study in all dose groups. Seventy-three out of the 142 subjects experienced a total of 209 adverse reactions (AR). Table 3 summarizes the most frequent ARs that occurred in more than 5% of subjects. Generally, the incidence of ARs was similar across the dose groups; the only AR where a dose effect was evident was headache, with an incidence of 2.9% in the 0.5 g/kg group, 14.5% in the 1.0 g/kg group and 23.7% in the 2.0 g/kg group. Pre-medication for AEs was only allowed in subjects who had adverse events during 2 consecutive infusions. During the study 11 subjects (7.75%) received premedication.
Table 3: Adverse Reactions* Occurring in more than 5% of Adult Subjects with CIDP
| Adverse Reaction | No. of Subjects with Adverse Reaction(percentage of subjects) |
| Headache | 21 (14.8 %) |
| Fever | 20 (14.1%) |
| Dermatitis | 14 (9.9%) |
| Blood Pressure Increased | 11 (7.7%) |
* Any infusional and any study medication related adverse events.
Two serious adverse reactions (headache and vomiting) were reported in one subject but did not lead to discontinuation of PANZYGA.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PANZYGA. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
|
Blood and lymphatic system disorders
Leucopenia, hemolysis, pancytopenia |
|
Immune system disorders
Hypersensitivity (e.g., anaphylaxis), anaphylactic shock, anaphylactic reaction, anaphylactoid reaction, allergic reaction, angioedema, face edema |
|
Metabolic and nutritional disorders
Fluid overload, (pseudo)hyponatremia |
|
Psychiatric disorders
Agitation, confusional state, anxiety, nervousness |
|
Nervous system disorders
Coma, loss of consciousness, seizures, (acute) encephalopathy, cerebrovascular accident, stroke, aseptic meningitis, migraine, speech disorder, paraesthesia, hypoesthesia, photophobia, tremor |
|
Cardiac disorders
Myocardial infarction, cardiac arrest, angina pectoris, tachycardia, bradycardia, palpitations, cyanosis |
|
Vascular disorders
Hypotension, (deep vein) thrombosis, peripheral circulatory failure/collapse, hypertension, phlebitis, pallor |
|
Respiratory, thoracic and mediastinal disorders
Apnea, Acute Respiratory Distress Syndrome (ARDS), TRALI, respiratory failure, pulmonary embolism, pulmonary edema, bronchospasm, dyspnea, hypoxia, wheezing, cough |
|
Gastrointestinal disorders
Diarrhea, hepatic dysfunction, abdominal discomfort |
|
Skin and subcutaneous tissue disorders
Eczema, urticaria, rash (erythematous), pruritus, alopecia, Stevens-Johnson syndrome, epidermolysis, skin exfoliation, erythema (multiforme), dermatitis (e.g., bullous dermatitis) |
|
Musculoskeletal and connective tissue disorders
Back pain, arthralgia, myalgia, musculoskeletal pain, muscle stiffness, pain in extremity, neck pain, muscle spasm |
|
Renal and urinary disorders
Acute renal failure, osmotic nephropathy, renal pain |
|
General disorders and administration site conditions
Injection site reaction, chills, chest pain or discomfort, hot flush, flushing, flu-like illness, feeling cold or hot, edema, hyperhidrosis, malaise, asthenia, lethargy, burning sensation |
|
Investigations
Hepatic enzymes increased, oxygen saturation decreased, falsely elevated erythrocyte sedimentation rate, positive direct antiglobulin (Coombs’) test |
Related/similar drugs
7. Drug Interactions
Clinical studies have not evaluated mixtures of PANZYGA with other drugs and intravenous solutions. It is recommended that PANZYGA is administered separately from other drugs or medications which the patient may be receiving. Do not mix the product.
Do not mix PANZYGA with IGIVs from other manufacturers.
Passively transferred antibodies in immunoglobulin preparations can confound the results of serological testing, e.g. false positive Treponema pallidum testing might occur.
Antibodies in PANZYGA may interfere with the response to live viral vaccines, such as measles, mumps, and rubella. Inform physicians of recent therapy with PANZYGA, so that administration of live viral vaccines, if indicated, can be appropriately delayed for 3 or more months from the time of PANZYGA administration.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
No human data are available to indicate the presence or absence of drug-associated risk. Animal reproduction studies have not been conducted with PANZYGA. It is also not known whether PANZYGA can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Immune globulins cross the placenta from maternal circulation increasingly after 30 weeks of gestation. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk summary
No human data are available to indicate the presence or absence of drug-associated risk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PANZYGA and any potential adverse effects on the breastfed infant from PANZYGA or from the underlying maternal condition.
8.4 Pediatric Use
Treatment of Primary Humoral Immunodeficiency (PI)
PANZYGA was evaluated in 25 pediatric subjects (age range: 2-15 years). Twenty-five percent of PI subjects exposed to PANZYGA were children (between 2 and 12 years of age). Pharmacokinetics, efficacy and safety were similar to those in adults. No specific dose requirements were necessary to achieve the targeted serum IgG levels in the pediatric subjects.
Treatment of Immune Thrombocytopenia (ITP) in children
The safety and effectiveness of PANZYGA have not been established in pediatric patients with ITP.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in children
The safety and effectiveness of PANZYGA have not been established in pediatric patients with CIDP.
8.5 Geriatric Use
Clinical studies of PANZYGA in PID and ITP did not include sufficient numbers of subjects older than 65 years to determine whether they respond differently from younger subjects.
In the clinical study the safety and effectiveness of PANZYGA in subjects with CIDP older than 65 years was similar to those 65 years of age and younger. A total of 36 subjects older than 65 years were included in the clinical trial.
Patients older than 65 years of age may be at increased risk for developing adverse reactions such as thromboembolic events and acute renal failure (See Boxed Warnings and Thrombotic Events ( 5.4 ) and Renal Failure ( 5.2 ) . Do not exceed recommended doses in this population, and apply the minimum practicable infusion rate.
10. Overdosage
With intravenous administration, overdose may lead to fluid overload and hyperviscosity. Patients at risk of complications of fluid overload and hyperviscosity include elderly patients and those with cardiac or renal impairment.
11. Panzyga Description
Immune Globulin Intravenous (Human), PANZYGA, is a solvent/detergent (S/D)-treated, sterile preparation of highly purified immunoglobulin G (IgG) derived from large pools of human plasma. PANZYGA is a solution for infusion to be administered intravenously.
This preparation contains approximately 100 mg of protein per mL (10%), of which not less than 96% is normal human immunoglobulin G. PANZYGA contains not more than 3% aggregates, not less than 90% monomers and dimers, and not more than 3% fragments. On average, the product contains 100 µg/mL of IgA, and lower amounts of IgM.
PANZYGA contains only trace amounts of sodium, and the pH is between 4.5 and 5.0. The osmolality is in the range of 240-310 mosmol/kg.
The manufacturing process for PANZYGA isolates IgG without additional chemical or enzymatic modification, and the Fc portion is maintained intact. PANZYGA contains the IgG antibody activities present in the donor population. IgG subclasses are fully represented with the following approximate percents of total IgG: IgG 1 is 65%, IgG 2 is 28%, IgG 3 is 3% and IgG 4 is 4%.
PANZYGA contains a broad spectrum of IgG antibodies against bacterial and viral agents that are capable of opsonization and neutralization of microbes and toxins. PANZYGA contains glycine (15.0-19.5 mg/mL), but no preservatives or sucrose.
All units of human plasma used in the manufacture of PANZYGA are provided by FDA-approved blood and plasma establishments, and are tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV and Nucleic Acid Test (NAT) for HCV and HIV1 and found to be non-reactive (negative).
The product is manufactured by the cold ethanol fractionation process followed by purification methodologies, as well as S/D treatment and nanofiltration (20 nm). The S/D mixture used is composed of tri-n-butyl phosphate (TNBP, solvent) and Triton X-100 (Octoxynol, detergent). The PANZYGA manufacturing process shows significant viral reduction and inactivation, demonstrated by in vitro infectivity studies ( Table 4 ). The virus safety of PANZYGA is achieved through a combination of various process steps, including S/D treatment, ion-exchange chromatography, and nanofiltration (20 nm).
Table 4 shows the virus clearance during the manufacturing process for PANZYGA, expressed as the mean log 10 reduction factor (LRF).
Table 4: Virus Reduction by PANZYGA Manufacturing Process
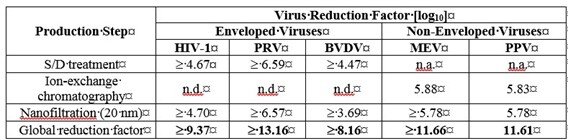
HIV-1: Human Immunodeficiency Virus – 1, a model for HIV-1 and HIV-2;
PRV: Pseudorabies Virus, a model for large enveloped DNA viruses (e.g., herpes virus);
BVDV: Bovine Viral Diarrhea Virus, a model for e.g., Hepatitis C virus (HCV) and West-Nile virus (WNV);
MEV: Mouse Encephalomyelitis virus, a model for Hepatitis A virus (HAV);
PPV: Porcine Parvovirus, a model for Human Parvovirus B19;
n.a.: not applicable;
n.d: not done.
Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents. [ 10 ]
Several of the individual production steps in the PANZYGA manufacturing process were shown to decrease TSE infectivity of that experimental model agent. TSE reduction steps include ion-exchange chromatography and nanofiltration, which together give a total of at least 10.4 log10 decrease of infectivity. These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
12. Panzyga - Clinical Pharmacology
12.1 Mechanism of Action
Treatment of Primary Humoral Immunodeficiency (PI)
PANZYGA supplies a broad spectrum of opsonic and neutralizing IgG antibodies against bacteria or their toxins. The mechanism of action in PI has not been fully elucidated.
Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
The mechanism of action of immunoglobulins in the treatment of chronic ITP in adults has not been fully elucidated.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
The mechanism of action of immunoglobulins in the treatment of CIDP in adults has not been fully elucidated.
12.2 Pharmacodynamics
PANZYGA contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. PANZYGA which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. Adequate doses of IGIV can restore abnormally low IgG level to the normal range. Standard pharmacodynamic studies were not performed.
12.3 Pharmacokinetics
Treatment of Primary Humoral Immunodeficiency (PI)
In the PI study, 50 pediatric and adult subjects underwent pharmacokinetic assessments. Subjects received infusions of PANZYGA (200 to 800 mg/kg body weight) every 3 or 4 weeks for 12 months. Blood samples for PK study were collected between the 7 th and 9 th PANZYGA infusion, depending on the individual treatment schedule.
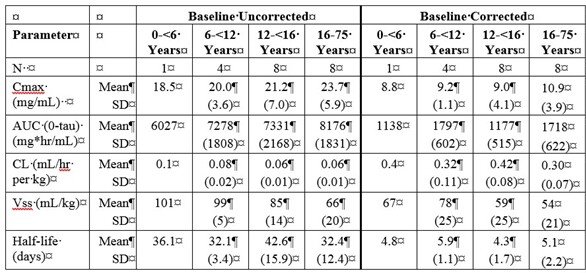
Table 5a and 5b summarize the pharmacokinetic parameters of PANZYGA, based on serum concentrations of total IgG, in subjects receiving infusions every 3, or 4 weeks, respectively.
Table 5: PI Study- Pharmacokinetic Parameters of PANZYGA in Subjects
a) PK Parameters: IgG Arm: 3-weeks
b) PK Parameters: IgG Arm: 4-weeks
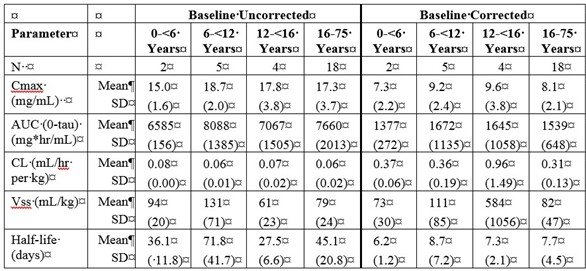
Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
Pharmacokinetic studies with PANZYGA have not been performed in adults with chronic ITP.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
Pharmacokinetic studies with PANZYGA have not been performed in adult patients with CIDP.
The IgG trough levels were evaluated at each visit ( Table 6 ) before the PANZYGA infusion. There was the option of rescue treatment with two consecutive infusions of 2.0 g/kg Panzyga at 3-week intervals (±4 days) for all subjects in the 0.5 and 1.0 g/kg Panzyga arms who were either stable at Week 6 or deteriorated after Week 3 and before Week 18. The actual administered dose for the three dose arms was: 0.91 ± 0.4 (n=35), 1.24 ± 0.2 (n=69) and 1.97 ± 0.2 (n=38) g/kg for 0.5, 1 and 2g/kg, respectively. There were no major differences among the three dose arms in demographic characteristics and baseline IgG levels. The percentage of increase in mean IgG from baseline to the end of study assessment was 46% in the 0.5 g/kg arm, 57% in the 1.0 g/kg arm and 91% in the 2.0 g/kg arm ( Table 6 ).
Table 6 Mean IgG trough levels in subjects with CIDP
| IgG troughs (g/L) | PANZYGA 0.5 g/kg | PANYZGA 1.0 g/kg | PANZYGA 2.0 g/kg | |
| Visit / Time point | (N=35) | (N=69) | (N=38) | |
| Visit 2 - Week 0 | Mean (SD) | 10.6 (3.1) | 10.5 (2.5) | 10.2 (3.0) |
| Visit 3 - Week 3 | Mean (SD) | 17.9 (3.5) | 17.1 (3.1) | 16.5 (3.3) |
| Visit 4 - Week 6 | Mean (SD) | 15.5 (3.0) | 16.5 (3.3) | 18.5 (4.0) |
| Visit 5 - Week 9 | Mean (SD) | 15.6 (3.3) | 16.5 (3.0) | 19.2 (4.4) |
| Visit 6 - Week 12 | Mean (SD) | 14.2 (2.6) | 16.8 (3.8) | 19.6 (4.3) |
| Visit 7 - Week 15 | Mean (SD) | 14.1 (2.7) | 16.2 (3.4) | 19.7 (4.4) |
| Visit 8 - Week 18 | Mean (SD) | 14.1 (2.3) | 15.9 (3.1) | 19.6 (5.2) |
| Visit 9 - Week 21 | Mean (SD) | 14.3 (2.3) | 16.0 (3.0) | 18.9 (3.5) |
| End of Study - Week 24 | Mean (SD) | 15.5 (3.6) | 16.5 (3.3) | 19.5 (4.6) |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies were conducted on carcinogenesis, mutagenesis, or impairment of fertility with PANZYGA.
13.2 Animal Toxicology and/or Pharmacology
Several standard nonclinical proof-of-concept and safety studies were performed with PANZYGA in animals. These included acute toxicity, pharmacokinetic, local tolerance, and safety pharmacology studies. PANZYGA. There were no adverse effects attributed to PANZYGA in the animal studies.
TNBP and Octoxynol-9 may be found in PANZYGA in trace amounts. In single- and repeated-dose toxicity studies in animals, these impurities caused no adverse effects when administered (alone or in combination) at doses multiple times higher than the equivalent human dose. A mixture of these compounds did not show teratogenic effects when administered to pregnant rabbits and rats during organogenesis.
14. Clinical Studies
14.1 Treatment of Primary Humoral Immunodeficiency (PI)
Study 1: In a prospective, open-label, single-arm, multicenter study in 51 children and adults with PI, subjects received PANZYGA at a dose between 200 to 800 mg/kg body weight every 3 or 4 weeks. Subjects participated in the study for a mean of 360 days. Infusions were initiated at a rate of 1 mg/kg/min for the first 30 minutes, and, if tolerated, could be advanced to a maximum tolerated rate not exceeding 8 mg/kg/min. The mean age of subjects was 26.8 years (range: 2 to 65 years).
The primary efficacy endpoint was the number of episodes of serious bacterial infections per patient per year. Serious infection included pneumonia, bacteremia or sepsis, osteomyelitis/septic arthritis, visceral abscesses, or bacterial meningitis. Secondary efficacy variables included: occurrence of any infection of any kind or seriousness; time to resolution of infections; use of antibiotics; the number of days of work/school missed; the number and days of hospitalizations; and the number of episodes of fever.
For the primary endpoint, the observed rate was 0.08 serious bacterial infections per patient per year (4 infections over 50.2 patient-years).
Only 1 adult patient was hospitalized due to an infection for 4 days (overall rate of days in hospital per person-year: 0.080). Episodes of fever were observed for less than 25% of all patients. The mean resolution time was 14 days for serious bacterial infections and 18 days for other infections. Approximately 50% of all patients missed at least 1 day of work or school due to infections, with an annual rate of less than 4 days/person-year.
Table 7 summarizes the efficacy results for all 51 subjects.
Table 7: Study 1 – Summary of Efficacy Results for subjects with PI
| Category | Result | Unit |
| Number of subjects | 51 | Subjects |
| Total number of subject days | 18,349 | Days |
| Annual rate of confirmed serious bacterial infections (SBIs)* | 0.080 | SBIs/person-year ** |
| Annual rate of other infections | 3.682 | Inf./person-year |
| Number of subjects (%) with use of antibiotics | 42 (82.4%) | Subjects(%) |
| Annual rate of use of antibiotics | 87 | Days/person-year |
| Absences from work or school due to Infection, number of days (%) | 183 (1.0%) | Days (%) |
| Annual rate of absences from work or school due to infection | 3.6 | Days/person-year |
| Hospitalization due to infection, number of days | 4 | Days |
| Annual rate of hospitalizations due to infection | 0.1 | Day/person-year |
* Defined as bacteremia/sepsis, bacterial meningitis, osteomyelitis/septic arthritis, bacterial pneumonia and visceral abscess
** Upper 1-sided 99% confidence interval: 0.503
Throughout the entire study, the serum IgG trough levels were nearly constant for both treatment schedules and were above the required trough levels of about 5-6 g/L. The calculated pharmacokinetic parameters showed that the minimum concentration of IgG was at least 6.8 g/L for both treatment intervals.
14.2 Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
A prospective, open-label, single-arm, multicenter study assessed the efficacy, safety, and tolerability of PANZYGA in 40 subjects with chronic ITP and a platelet count of 20 x 10 9 /L or less. Subjects ranged in age from 18 to 72 years (median: 32 years); 43% were female and 57% were male. Ninety percent of the subjects were Caucasian and 10% were Asian.
Subjects received a 2 g/kg dose of PANZYGA administered as two daily 1 g/kg intravenous doses, given on 2 consecutive days. All but one patient received the maximum infusion rate of 8 mg/kg/minute, starting at 1 mg/kg/minute. Platelet counts were measured on Days 1 to 8, 15, and 22.
The study was designed to determine the response rate, defined as the percentage of subjects with an increase in platelet count to at least 50 x 10 9 /L within 7 days after the first infusion (responders). Additionally, maximum platelet count, the time to reach a platelet count of at least 50 x 10 9 /L within the first 7 days, the duration of that response (i.e., the number of days the platelet count remained in excess of 50 x 10 9 /L), and the regression of hemorrhages in subjects who had bleeding at baseline were observed.
Of the 36 subjects in the full analysis set, 29 (81%: 95% CI: 64%- 92%).) responded to PANZYGA with a rise in platelet count to at least 50 x 10 9 /L within 7 days after the first infusion. The lower bound of the overall 95% confidence interval for the response rate in all 36 subjects (64%) is above the predefined response rate of 60%.
Table 8 shows the median and mean of the maximum platelet count.
Table 8: Maximum Platelet Count (x10 9 /L)
| ITP subjects (n=36) | |
| Median and range | 196 (8 to 1067) |
| Mean ± standard deviation | 237 ± 205 |
Table 9 shows the median and mean of the time to and duration of platelet response.
Table 9: Time to and Duration of Platelet Response (Responders Only).
| Time to Platelet Response (at least 50x10 9 /L) (Days) | Duration of Platelet Response (Days) | |
| ITP Subjects Responders (n=29) | ITP Subjects Responders (n=29) | |
| Median and range | 2 (1 to 4) | 14 (1 to 20) |
| Mean ± standard deviation | 1.8 ± 0.8 | 12.4 ± 5.8 |
Of the 36 subjects, 23 (64%) subjects had bleeding at baseline. Bleeding was minor in 14 subjects (39%), mild in 2 subjects (6%) and moderate in 7 subjects (19%). On Day 7, only 14% of subjects were bleeding (5/36). Persistent bleeding was mild in 1 and minor in 2 subjects. Information regarding bleeding resolution was missing in 2 subjects with moderate bleeding.
14.3 Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
The efficacy of PANZYGA in adults with CIDP was evaluated in a prospective, double-blind, randomized, multicenter study that enrolled 142 adult subjects (between 18 and 83 years of age) with CIDP who deteriorated in the Wash-out Phase, during which the current medication (immunoglobulins or corticosteroids) was reduced gradually. Subjects were randomized 1:2:1 to receive first a loading dose of 2 g/kg, and then 0.5 g/kg, 1.0 g/kg or 2.0 g/kg PANZYGA according to their respective dose arm for 7 maintenance infusions at 3-week intervals during the 24-week Dose-evaluation Phase. Subjects in the 0.5 g/kg and 1.0 g/kg arms had the option of rescue treatment with two consecutive infusions of 2.0 g/kg Panzyga at 3-week intervals if criteria were met [ CLINICAL PHARMACOLOGY (12.3)].
Efficacy was based on the proportion of responders in the 1.0 g/kg PANZYGA arm at Week 24 relative to Baseline (Week 0). A responder was defined as a subject with a decrease of at least 1 point in the adjusted 10-point Inflammatory Neuropathy Cause and Treatment (INCAT) disability score at Week 24 relative to Baseline. The proportion of responders in the 1.0 g/kg arm was 79.71% (95% CI: 68.8, 87.5), with 55 out of 69 subjects classified as responders. Efficacy was supported by the proportion of responders in the 2.0 g/kg dose arm in the adjusted INCAT disability score, and the proportion of responders in the 1.0 g/kg and 2.0 g/kg dose arms in the grip strength, inflammatory Rasch-built Overall Disability Scale (I-RODS) and Medical Research Council (MRC) sum scores ( Table 10 ).
Table 10: Responder rates for different efficacy scores and dose arms
|
1.0 g/kg
N=69 |
2.0 g/kg
N=36 |
|
| Adjusted INCAT Disability Score | ||
| Number (%) of responders | 55 (79.7%) | 33 (91.7%) |
| 95% CI | 68.8; 87.5 | 78.2; 97.1 |
| I-RODS | ||
| Number (%) of responders | 38 (55.1%) | 26 (72.2%) |
| 95% CI | 43.4; 66.2 | 56; 84.2 |
| Grip Strength | ||
| Number (%) of responders | 45 (65.2%) | 30 (83.3%) |
| 95% CI | 53.4; 75.4 | 68.1; 92.1 |
| MRC Sum Score | ||
| Number (%) of responders | 50 (72.5%) | 31 (86.1%) |
| 95% CI | 61; 81.6 | 71.3; 93.9 |
15. References
- Duhem C, Dicato MA, Ries F: Side-effects of intravenous immune globulins. Clin.Exp.Immunol. 1994;97 Suppl 1:79-83.
- Steinberger BA, Ford SM, Coleman TA: Intravenous immunoglobulin therapy results in post-infusional hyperproteinemia, increased serum viscosity, and pseudohyponatremia. Am.J Hematol. 2003;73:97-100.
- Dalakas MC: High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994;44:223-226.
- Go RS, Call TG: Deep venous thrombosis of the arm after intravenous immunoglobulin infusion: case report and literature review of intravenous immunoglobulin-related thrombotic complications. Mayo Clin Proc 2000;75:83-85.
- Wolberg AS, Kon RH, Monroe DM, et al: Coagulation factor XI is a contaminant in intravenous immunoglobulin preparations. Am.J.Hematol. 2000;65:30-34.
- Sekul EA, Cupler EJ, Dalakas MC: Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors. Ann Intern.Med 1994;121:259-262.
- Kahwaji J, Barker E, Pepkowitz S, et al: Acute Hemolysis After High-Dose Intravenous Immunoglobulin Therapy in Highly HLA Sensitized Patients. Clin J Am Soc Nephrol 2009;4:1993-1997.
- Daw Z, Padmore R, Neurath D, et al: Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: A case series analysis. Transfusion 2008;48:1598-1601.
- Rizk A, Gorson KC, Kenney L, et al: Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 2001;41:264-268.
- Radomski KU, Lattner G, Schmidt T, Römisch J: Pathogen Safety of a New Intravenous Immune Globulin 10% Liquid. BioDrugs 2017(2): 125-134
16. How is Panzyga supplied
PANZYGA is supplied in 1 g, 2.5 g, 5 g, 10 g, 20 g, and 30 g single-use bottles.
The table below shows the details of available presentations of PANZYGA.
| Carton NDC Number | Container NDC Number | Size | Grams Protein |
| 0069-1011-02 | 0069-1011-01 | 10 mL | 1 |
| 0069-1109-02 | 0069-1109-01 | 25 mL | 2.5 |
| 0069-1224-02 | 0069-1224-01 | 50 mL | 5 |
| 0069-1312-02 | 0069-1312-01 | 100 mL | 10 |
| 0069-1415-02 | 0069-1415-01 | 200 mL | 20 |
| 0069-1558-02 | 0069-1558-01 | 300 mL | 30 |
PANZYGA is not supplied with an infusion set. If a filtered infusion set is used (not mandatory), choose a filter size of 0.2-200 microns.
Components used in the packaging of PANZYGA are not made with natural rubber latex.
Store PANZYGA for 36 months at +2°C to +8°C (36°F to 46°F) from the date of manufacture. Within its shelf-life, the product may be stored at ≤ +25°C (77°F) for up to 12 months. After storage at ≤ +25°C (77°F), either use immediately or discard the product.
Do not use after expiration date.
Do not freeze. Do not use frozen product.
PANZYGA contains no preservatives. The PANZYGA bottle is for single use only. Use promptly any bottle that has been entered or opened, and discard partially used bottles.
Dispose of any unused product or waste material in accordance with local requirements.
17. Patient Counseling Information
Inform patients of the signs and symptoms of hypersensitivity reactions including urticaria, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis, and to contact their physicians immediately if allergic symptoms occur.
Inform patients to immediately report the signs and symptoms of the following conditions to their physician:
- Decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath, which may suggest kidney problems (see Renal Failure ( 5.2 )) .
- Symptoms of thrombosis which may include: pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body (see Thrombosis ( 5.4 ))
- Severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea and vomiting (see Aseptic Meningitis Syndrome ( 5.5 ))
- Increased heart rate, fatigue, yellowing of skin or eyes and dark-colored urine (see Hemolysis ( 5.6 ))
- Trouble breathing, chest pain, blue lips or extremities, fever (see TRALI ( 5.7 ))
Inform patients that PANZYGA is made from human plasma and may contain infectious agents that can cause disease (e.g., viruses, and theoretically, the CJD agent), and that the risk of infectious agent transmission has been reduced by (a) screening plasma donors for prior exposure to viruses, (b) testing the donated plasma for viral infections and (c) inactivating and/or removing viruses during manufacture.
Inform patients that administration of PANZYGA may interfere with the response to live viral vaccines such as measles, mumps and rubella, and to notify their immunizing physician of their therapy with PANZYGA.
Manufactured by:
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
1100 Vienna, Austria

Distributed by:
Pfizer Labs
Division of Pfizer Inc
New York, NY 10001
18 PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL
Immune Globulin Intravenous (Human) - ifas, 10%
Panzyga
10 mL
NDC 0069-1011-02
Carton
Panzyga
25 mL
NDC 0069-1109-02
Carton
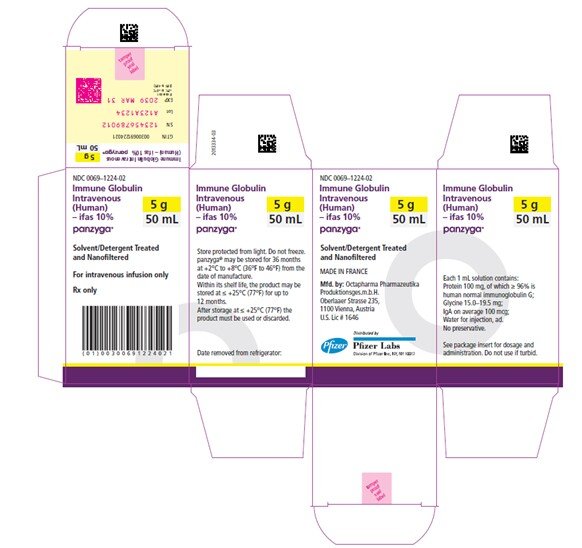
Panzyga
50 mL
NDC 0069-1224-02
Carton
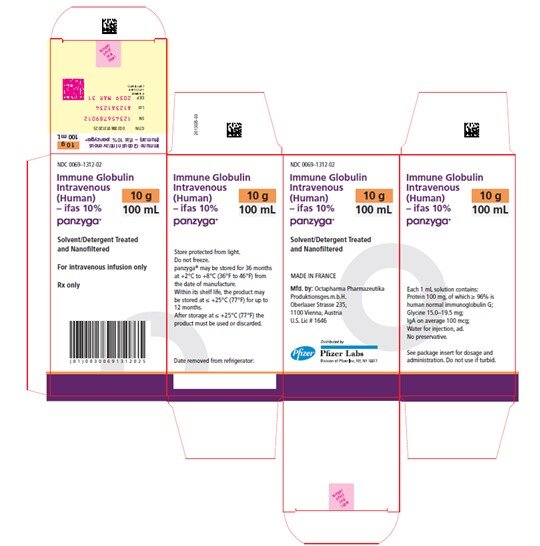
Panzyga
100 mL
NDC 0069-1312-02
Carton

Panzyga
200 mL
NDC 0069-1415-02
Carton
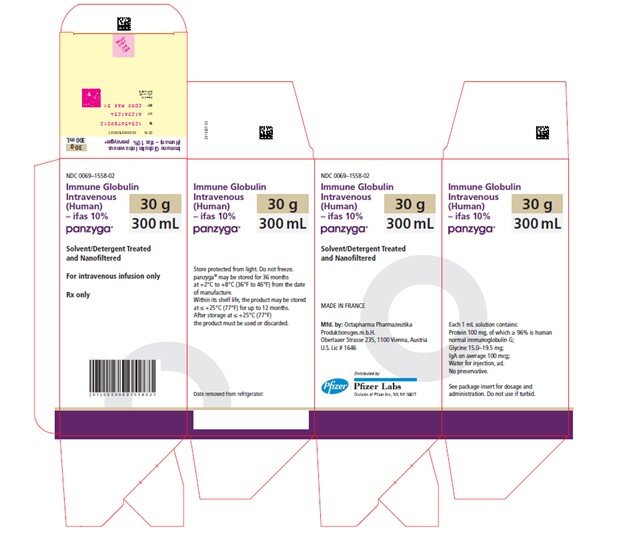
Panzyga
300 mL
NDC 0069-1558-02
Carton
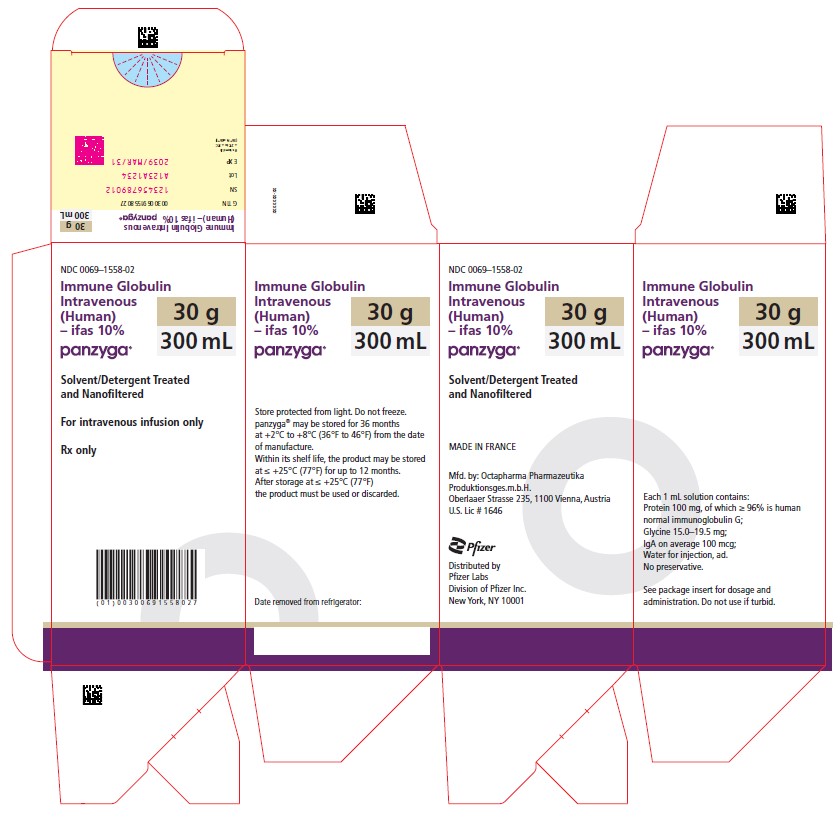
| PANZYGA
immune globulin intravenous (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PANZYGA
immune globulin intravenous (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PANZYGA
immune globulin intravenous (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PANZYGA
immune globulin intravenous (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PANZYGA
immune globulin intravenous (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PANZYGA
immune globulin intravenous (human) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
More about Panzyga (immune globulin intravenous)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (4)
- Latest FDA alerts (2)
- Side effects
- Dosage information
- During pregnancy
- Drug class: immune globulins
- En español
Patient resources
Professional resources
Other brands
Octagam, Privigen, Gammaplex, Bivigam, ... +6 more
