Altuviiio: Package Insert / Prescribing Info
Package insert / product label
Generic name: antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl
Dosage form: injection kit
Drug class: Miscellaneous coagulation modifiers
J Code (medical billing code): J7214 (Per intl units, injection)
Medically reviewed by Drugs.com. Last updated on Mar 17, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl], lyophilized powder for solution, for intravenous use
Initial U.S. Approval: 2023
Recent Major Changes
| Dosage and Administration (2.3) | 05/2024 |
Indications and Usage for Altuviiio
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment & control of bleeding episodes
- Perioperative management of bleeding (1)
Limitation of Use:
ALTUVIIIO is not indicated for the treatment of von Willebrand disease. (1)
Altuviiio Dosage and Administration
For intravenous use only.
- Each ALTUVIIIO vial label states Factor VIII activity in international units (IU or unit). (2.1)
- For routine prophylaxis: 50 IU/kg once weekly. (2.1)
- For on-demand treatment and control of bleeding episodes and perioperative management: 50 IU/kg (2.1)
Estimated Increment of Factor VIII (IU/dL or % of normal) = 50 IU/kg × 2 (IU/dL per IU/kg) (2.1)
To achieve a specific target Factor VIII activity level, use the following formula: Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL). (2.1)
Dosage Forms and Strengths
For injection: nominally 250, 500, 750, 1000, 2000, 3000, or 4000 IU, lyophilized powder in single-dose vials for reconstitution. (3)
Contraindications
Do not use in patients who have had severe hypersensitivity reactions, including anaphylaxis, to ALTUVIIIO or excipients of ALTUVIIIO. (4)
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, have occurred with ALTUVIIIO. If symptoms occur, immediately discontinue ALTUVIIIO and initiate appropriate treatment. (5.1)
- Neutralizing antibodies (inhibitors) to Factor VIII have been reported. If expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures Factor VIII inhibitor concentration. (5.2, 5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence >10%) are headache and arthralgia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bioverativ Therapeutics Inc. (A SANOFI COMPANY) at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
Full Prescribing Information
1. Indications and Usage for Altuviiio
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a von Willebrand Factor (VWF) independent recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
2. Altuviiio Dosage and Administration
For intravenous use after reconstitution only.
2.1 Dose
- Each ALTUVIIIO vial label states the Factor VIII potency in international units (IU). One IU corresponds to the Factor VIII activity contained in one milliliter of normal human plasma, as defined by the current World Health Organization (WHO) international standard for Factor VIII concentrate.
- Potency assignment for ALTUVIIIO is determined using an activated partial thromboplastin time (aPTT)-based one-stage clotting assay. It is recommended to use a validated one-stage clotting assay to measure ALTUVIIIO Factor VIII activity in plasma. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid based aPTT reagent in one-stage clotting assay by approximately 2.5-fold [see Warnings and Precautions (5.3)].
For the dose of 50 IU/kg, the expected in vivo peak increase in Factor VIII level expressed as IU/dL (or % of normal) is estimated using the following formula:
Estimated Increment of Factor VIII (IU/dL or % of normal) = 50 IU/kg × 2 (IU/dL per IU/kg)
To achieve a specific target Factor VIII activity level, use the following formula: Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL).
Routine Prophylaxis
The recommended dosing for routine prophylaxis for adults and children is 50 IU/kg of ALTUVIIIO administered once weekly.
On-demand Treatment and Control of Bleeding Episodes
ALTUVIIIO dosing for the on-demand treatment and control of bleeding episodes is provided in Table 1.
| Type of Bleeding | Recommended Dose | Additional Information |
|---|---|---|
| Minor and Moderate
For example: Uncomplicated joint bleeds, minor muscular bleeds, mucosal or subcutaneous bleeds | Single dose of 50 IU/kg | For minor and moderate bleeding episodes occurring within 2 to 3 days after a prophylactic dose, a lower dose of 30 IU/kg dose may be used. Additional doses of 30 or 50 IU/kg every 2 to 3 days may be considered. |
| Major
For example: Intracranial, retroperitoneal, iliopsoas and neck bleeds, muscle bleeds with compartment syndrome and bleeds associated with a significant decrease in the hemoglobin level | Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days can be considered. |
For resumption of prophylaxis (if applicable) after treatment of a bleed, it is recommended to allow an interval of at least 72 hours between the last 50 IU/kg dose for treatment of a bleed and resuming prophylaxis dosing. Thereafter, prophylaxis can be continued as usual on the patient's regular schedule.
Perioperative Management
ALTUVIIIO dosing for perioperative management is provided in Table 2.
| Type of Surgery | Pre-operative Dose | Post-operative Dose |
|---|---|---|
| Minor
For example: Tooth extraction | Single dose of 50 IU/kg | An additional dose of 30 or 50 IU/kg after 2 to 3 days may be considered. |
| Major
For example: Intracranial, intra-abdominal, joint replacement surgery, or complicated dental procedures. | Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days may be administered as clinically needed for perioperative management. |
2.2 Preparation and Reconstitution
- Use aseptic technique and a flat work surface during the reconstitution procedure.
- Allow the ALTUVIIIO vial, containing the white to off-white lyophilized powder, and the prefilled diluent syringe to reach room temperature before use.
- Remove the plastic cap from the ALTUVIIIO vial and wipe the rubber stopper of the vial with an alcohol wipe. Allow the rubber stopper to dry.
- Completely remove the backing from the vial adapter package by peeling back the lid. Do not remove the vial adapter from the package or touch the inside of the package of the adapter.

- Keep the vial on a flat surface. Hold the vial with one hand and using the other hand, place the vial adapter in its package over the vial. The spike should be placed directly above the center of the rubber stopper. Push the vial adapter straight down until the spike on the vial adapter punctures the center of the vial stopper and is fully inserted.
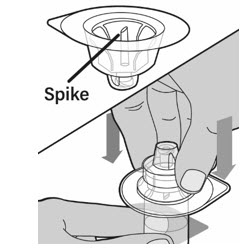
- Lift the package cover away from the vial adapter and throw away the cover.
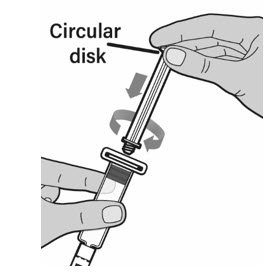
- Only use the prefilled diluent syringe provided to reconstitute the powdered medicine. Hold the plunger rod by the circular disk. Place the tip of the plunger rod into the end of the prefilled diluent syringe. Turn the plunger rod to the right until it is firmly attached.
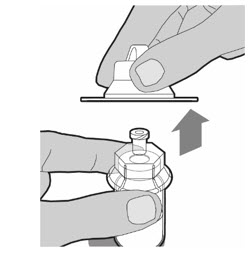
- With one hand, hold the prefilled diluent syringe directly under the cap with the cap pointing up. Make sure you are holding the prefilled diluent syringe by the ridged part directly under the cap. Do not use if the cap has been removed or is not securely attached.
- With your other hand, grasp the cap and bend it at a 90 degree angle until it snaps off. After the cap snaps off, you will see the glass tip of the prefilled diluent syringe. Do not touch the glass tip of the prefilled diluent syringe or the inside of the cap.
- Be sure the vial is sitting on a flat surface. Insert the tip of the prefilled diluent syringe into the vial adapter opening. Turn the prefilled diluent syringe to the right until it is securely attached to the vial adapter.
- Slowly push down on the plunger rod to inject all of the liquid (diluent) from the prefilled diluent syringe into the vial. The plunger rod may rise slightly afterward. This is normal.
- With the prefilled diluent syringe still connected to the adapter, gently swirl the vial until the powder is completely dissolved. Check the solution through the vial to make sure the powder is fully dissolved. The solution should look clear and colorless to opalescent. Do not shake. Do not use the reconstituted ALTUVIIIO if it contains visible particles or is cloudy.
POOLING: pooling is the process of combining two or more reconstituted vials into a larger luer lock syringe (not provided in the carton). If the dose requires more than one vial, reconstitute each vial as described above (See Steps 3–12) with the prefilled diluent syringe provided. Do not detach the prefilled diluent syringe until you are ready to attach the larger luer lock syringe to the next vial. Keep the vial adapter attached to the vial as you will need it for attaching a larger luer lock syringe. Use a larger luer lock plastic syringe to combine the contents of the reconstituted vials into the syringe, similar as described in Steps 13–14. Repeat this pooling procedure with each vial you will be using. Once you have pooled the required dose, proceed with the administration steps using the larger luer lock syringe. - Make sure the plunger rod is pressed all the way down and the diluent syringe is firmly attached to the vial adapter. Turn the vial upside-down. Slowly pull down on the plunger rod to draw all the solution from the vial into the diluent syringe. Be careful not to pull the plunger rod completely out of the diluent syringe.
- Gently unscrew the diluent syringe from the vial adapter by turning it to the right. Dispose of the vial with the adapter still attached. If you are not ready to inject, put the syringe cap carefully back onto the syringe tip. Do not touch the syringe tip or the inside of the cap.
- Use the reconstituted ALTUVIIIO as soon as possible, but no later than 3 hours after reconstitution. Do not touch the glass tip of the syringe if not used immediately after reconstitution. Protect from direct sunlight. Do not refrigerate after reconstitution.
2.3 Administration
For intravenous use only.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use ALTUVIIIO solution if particulate matter or discoloration is observed.
- Do not administer reconstituted ALTUVIIIO in the same tubing or container with other medications.
Administration Steps:
- Attach the syringe to the connector end of the infusion set tubing by turning it to the right until it is securely attached.
- Push the plunger rod until all air is removed from the syringe and ALTUVIIIO has filled the infusion set needle. Do not push ALTUVIIIO solution through the needle.
- Remove the protective needle cover from the infusion set needle.
- Perform intravenous injection slowly over 1 to 10 minutes, based on the patient's comfort level.
- After infusing ALTUVIIIO, remove and properly discard the infusion set.
3. Dosage Forms and Strengths
ALTUVIIIO is available as a white to off-white lyophilized powder for reconstitution in single-dose vials containing nominally 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU) per vial.
4. Contraindications
ALTUVIIIO is contraindicated in patients who have had severe hypersensitivity reactions, including anaphylaxis, to the product or its excipients [see Description (11)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have occurred with ALTUVIIIO [see Postmarketing Experience (6.2)]. Signs and symptoms include, but not limited to, hives, shortness of breath, chest tightness, wheezing, hypotension, nausea, vomiting, and itching. Discontinue ALTUVIIIO if hypersensitivity reaction occurs and manage symptoms as appropriate.
5.2 Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to Factor VIII is possible following administration of ALTUVIIIO. Neutralizing antibodies were not reported in the clinical trials. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If the patient's plasma Factor VIII level fails to increase as expected or if bleeding is not controlled after ALTUVIIIO administration, the presence of an inhibitor (neutralizing antibodies) should be suspected, and appropriate testing performed [see Warnings and Precautions (5.3)].
5.3 Monitoring Laboratory Tests
If assessment of plasma Factor VIII activity is needed, it is recommended to use a validated one-stage clotting assay [see Dosage and Administration (2)]. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid based aPTT reagent in one-stage clotting assay by approximately 2.5-fold. If these assays are used, divide the result by 2.5 to approximate the patient's ALTUVIIIO Factor VIII activity level. Use of a reference laboratory is recommended when a qualified one-stage clotting assay or chromogenic assay is not available locally.
Monitor for the development of Factor VIII inhibitors. If bleeding is not controlled with ALTUVIIIO and the expected factor VIII activity plasma levels are not attained, perform an assay to determine if Factor VIII inhibitors are present (use Bethesda Units to titer inhibitors).
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ALTUVIIIO has been evaluated in 159 previously treated patients (PTPs) (134 adults and 25 adolescents) with severe Hemophilia A (<1% endogenous FVIII activity or a genetic mutation consistent with severe Hemophilia A) who received at least one dose of ALTUVIIIO for either routine prophylaxis, on-demand treatment of bleeding episodes or perioperative management. A total of 152 (96%) patients achieved at least 25 exposure days and 115 (72%) patients achieved at least 50 exposure days with a median of 53.0 (range 2–63) for both exposure days and injections per patient. Overall exposure was monitored for a total of 151.5 patient-years.
In the pediatric study, the safety of ALTUVIIIO was evaluated in 74 male PTPs <12 years of age with severe hemophilia A who received at least one dose of ALTUVIIIO. Sixty-six (89.2%) patients achieved at least 50 exposure days with a median of 53.0 (range 3–72).
Adverse events were monitored for a total of 210.7 patient-years in 2 completed clinical studies in PTPs. Adverse drug reactions (ADRs) (summarized in Table 3) were reported in 79 (33.9%) of the 233 patients treated with routine prophylaxis or on-demand therapy. The most common ADRs (>10%) in adults and adolescents were headache (20.1%) and arthralgia (16.4%). In children below 12 years, pyrexia (12.2%) was the most common ADR (>10%). In the studies, no inhibitors to FVIII were detected and no ADRs of anaphylaxis were reported.
The most common adverse reactions (>10% of patients) reported in clinical trials were headache and arthralgia.
| MedDRA System Organ Class | Adverse Drug Reactions | Number of Patients n (%) (N = 233)* |
|---|---|---|
|
||
| Nervous system disorders | Headache | 35 (15) |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 31 (13) | |
| Pain in extremity | 10 (4) | |
| Back pain | 9 (4) | |
| General disorders and administration | Pyrexia | 10 (4) |
| Gastrointestinal disorders | Vomiting | 7 (3) |
Thromboembolic events occurred in 1% (3/261) of patients in the long-term safety extension study; these three patients had pre-existing risk factors.
Immunogenicity
All patients were monitored for neutralizing antibodies (inhibitors) to Factor VIII in the clinical program. No patients developed neutralizing antibodies to Factor VIII [see Immunogenicity (12.6)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during the post approval use of ALTUVIIIO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Hypersensitivity reactions, including anaphylaxis [see Warnings and Precautions (5.1)].
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no data with ALTUVIIIO use in pregnant women to inform a drug-associated risk. Animal developmental and reproductive studies have not been conducted with ALTUVIIIO. Therefore, it is not known whether ALTUVIIIO can affect reproductive capacity or cause fetal harm when given to pregnant women.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ALTUVIIIO in human milk, its effects on the breastfed infant, or its effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ALTUVIIIO and any potential adverse effects on the breastfed infant from ALTUVIIIO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ALTUVIIIO have been established in pediatric patients <18 years old. Safety, efficacy, and pharmacokinetics (PK) have been evaluated in 99 previously treated patients <18 years of age who received at least one dose of ALTUVIIIO as part of routine prophylaxis, treatment of bleeding episodes, or perioperative management. Adolescents were enrolled in the adult and adolescent study and children <12 years of age were enrolled in the pediatric trial. Thirty-eight patients (38.4%) were <6 years of age, 36 (36.4%) patients were 6 to <12 years of age, and 25 patients (25.2%) were adolescents (12 to <18 years of age). Data from the pediatric study (74 patients) <12 years of age showed that no dosing adjustment was required [see Clinical Pharmacology (12)].
8.5 Geriatric Use
Clinical studies of ALTUVIIIO did not include sufficient numbers of patients 65 years of age and older to determine whether or not such patients respond differently from younger patients. However, clinical experience with other Factor VIII products has not identified differences between the elderly and younger patients.
11. Altuviiio Description
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution for intravenous injection. The product is supplied in single-dose vials containing nominal potencies of 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU). Each vial of ALTUVIIIO is labeled with the actual Factor VIII activity content in IU. The powder for injection is reconstituted with 3 mL sterile water for injection (sWFI) supplied in a sterile prefilled syringe. The reconstituted solution should be essentially free of particles. The final product contains the excipients: arginine hydrochloride (250 mM), calcium chloride dihydrate (5 mM), histidine (10 mM), polysorbate 80 (0.05% w/v), and sucrose (5% w/v).
The active ingredient in ALTUVIIIO is a fully recombinant fusion protein comprising a single chain B-domain deleted (BDD) analogue of human FVIII covalently fused to the Fc domain of human immunoglobulin G1 (IgG1), the FVIII-binding D'D3 domain of human von Willebrand factor (VWF), and 2 XTEN polypeptides. ALTUVIIIO contains 2829 amino acids with an apparent molecular weight of 312 kDa. ALTUVIIIO is synthesized as 2 polypeptide chains which are covalently linked by 2 Fc hinge disulfide bonds. The first FVIII-XTEN-Fc polypeptide chain contains the A1A2 domain of FVIII along with 5 amino acids from B-domain (1–745 amino acids) fused to the 288-XTEN polypeptide (in place of the natural FVIII B-domain), the A3C1C2 domain of FVIII (1649–2332), and the Fc domain of human IgG1. The second VWF-XTEN-a2-Fc polypeptide chain contains the D'D3 domain of VWF (1–477 amino acids) fused to the 144-XTEN polypeptide, a thrombin cleavable acidic region 2 sequence from FVIII and the Fc domain of human IgG1. The Fc domain includes the hinge, CH2, and CH3 domains of IgG1. The Fc, VWF, and XTEN polypeptide portions of the molecule extend the half-life of ALTUVIIIO in plasma.
ALTUVIIIO is produced by recombinant DNA technology in a human embryonic kidney (HEK) cell line, which has been extensively characterized. ALTUVIIIO is manufactured without addition of human- or animal-derived components and purified by a combination of multiple chromatography steps, a detergent viral inactivation step, a nano filtration step for viral clearance, and ultrafiltration steps.
12. Altuviiio - Clinical Pharmacology
12.1 Mechanism of Action
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] temporarily replaces the missing coagulation factor VIII needed for effective hemostasis. ALTUVIIIO has demonstrated 3- to 4-fold prolonged half-life relative to other standard and extended half-life FVIII products.
Mechanism of Half-life Extension
ALTUVIIIO is a recombinant FVIII analogue fusion protein that is independent of endogenous VWF in order to overcome the half-life limit imposed by FVIII-VWF interactions. The D'D3 domain of VWF is the region that interacts with FVIII. Appending the D'D3 domain of VWF to a recombinant FVIII-Fc fusion protein provides protection and stability to FVIII, and prevents FVIII interaction with endogenous VWF, thus overcoming the limitation on FVIII half-life imposed by VWF clearance.
The Fc region of human immunoglobulin G1 (IgG1) binds to the neonatal Fc receptor (FcRn). FcRn is part of a naturally occurring pathway that delays lysosomal degradation of immunoglobulins by recycling them back into circulation, thus prolonging the plasma half-life of the fusion protein.
ALTUVIIIO contains 2 XTEN polypeptides, which alter the hydrodynamic radius of the fusion protein, thus reducing rates of clearance and degradation, and improving pharmacokinetic properties. In ALTUVIIIO, the natural FVIII B domain (except 5 amino acids) is replaced with the first XTEN polypeptide, inserted in between FVIII N745 and E1649 amino acid residues; and the second XTEN polypeptide is inserted in between the D'D3 domain and Fc.
12.2 Pharmacodynamics
Hemophilia A is a bleeding disorder characterized by a deficiency of functional coagulation factor VIII (FVIII), which leads to a prolonged clotting time in the activated partial thromboplastin time (aPTT)-based one-stage clotting assay. Administration of ALTUVIIIO increases plasma levels of FVIII, temporarily correcting the coagulation defect in hemophilia A patients.
Based on FVIII pharmacokinetic/pharmacodynamic analyses, the risk of bleeding is negatively correlated with FVIII activity. Once weekly 50 IU/kg ALTUVIIIO provided factor VIII activity levels that were associated with a low bleed risk.
12.3 Pharmacokinetics
The PK of ALTUVIIIO were evaluated in prospective, open-label clinical studies, enrolling 159 adults and adolescents, and 74 children <12 years old, respectively, receiving weekly IV injections of 50 IU/kg. Among children <12 years old, 37 patients had ALTUVIIIO single dose PK profiles available.
PK parameters following a single dose of ALTUVIIIO are presented in Table 4. The PK parameters were based on plasma FVIII activity measured by the aPTT-based one-stage clotting assay. After a single dose of 50 IU/kg, ALTUVIIIO exhibited high sustained FVIII activity with prolonged half-life across age cohorts. There was a trend of increasing area under the curve (AUC), and decreasing clearance, with increasing age in the pediatric cohorts. The PK profile at steady state (Week 26) was comparable with the PK profile obtained after the first dose.
| PK Parameters (mean SD) | Pediatric Study | Pediatric Study | Adult and Adolescent Study | Adult and Adolescent Study |
|---|---|---|---|---|
| 1 to <6 Years N = 18 | 6 to <12 Years N = 18 | 12 to <18 years N = 25 | Adults N = 134 |
|
| AUC0–tau = area under the activity-time curve over the dosing interval, CL = clearance, MRT = mean residence time, SD = standard deviation, t1/2z = terminal half-life, Vss = volume of distribution at steady state. | ||||
| AUC (IU×h/dL) | 6800 (1120)* | 7190 (1450) | 8350 (1550) | 9850 (2010)† |
| t1/2 (h) | 38.0 (3.7) | 42.4 (3.7) | 44.6 (5.0) | 48.2 (9.3) |
| CL (mL/h/kg) | 0.742 (0.121) | 0.681 (0.139) | 0.582 (0.115) | 0.493 (0.121)† |
| Vss (mL/kg) | 36.6 (5.6) | 38.1 (6.8) | 34.9 (7.4) | 31.0 (7.3)† |
| MRT (hr) | 49.6 (5.5) | 56.3 (5.1) | 60.0 (5.5) | 63.9 (10.2)† |
ALTUVIIIO at steady state maintained normal to near normal (>40 IU/dL) FVIII activity for a mean (SD) of 4.1 (0.7) days with once weekly prophylaxis in adults. The FVIII activity over 10 IU/dL was maintained in 83.5% of adults and adolescent patients throughout the study. In children <12 years ALTUVIIIO maintained normal to near normal (>40 IU/dL) FVIII activity for 2 to 3 days and >10 IU/dL FVIII activity for approximately 7 days (see Table 5).
| PK Parameters Mean (SD) | Pediatric Study* | Pediatric Study* | Adult and Adolescent Study* | Adult and Adolescent Study* |
|---|---|---|---|---|
| 1 to <6 years N = 37 | 6 to <12 years N = 36 | 12 to <18 years N = 24 | Adults N = 125 |
|
| Peak = 15 min post dose at steady state, IR = incremental recovery, Trough – predose FVIII activity value at steady state, SD = standard deviation. | ||||
| Peak (IU/dL) | 136 (49) (N = 35) | 131 (36) (N = 35) | 124 (31) | 150 (35) (N = 124) |
| IR (kg×IU/dL/IU) | 2.22 (0.83) (N = 35) | 2.10 (0.73) (N = 35) | 2.25 (0.61) (N = 22) | 2.64 (0.61) (N = 120) |
| Time to 40 IU/dL (h) | 68.0 (10.5)† | 80.6 (12.3)† | 81.5 (12.1)‡ | 98.1 (20.1)‡ |
| Time to 20 IU/dL (h) | 109 (14)† | 127 (15)† | 130 (16)‡ | 150 (28)‡ |
| Time to 10 IU/dL (h) | 150 (18)† | 173 (17)† | 179 (20)‡ | 201 (36)‡ |
| Trough (IU/dL) | 10.9 (19.7) (N = 36) | 16.5 (23.7) | 9.23 (4.77) (N = 22) | 18.0 (16.6) (N = 123) |
Specific Populations
The following factors have no clinically meaningful effect on the pharmacokinetics of ALTUVIIIO: age (1.4 to 72 years), sex, race (White, Asian), VWF activity (40 to 339 IU/dL), hematocrit level (28% to 57%), blood type, HCV status, or HIV status. Body weight (12.5 to 133 kg) is expected to alter weight normalized clearance (dL/h/kg) by 79% to -18% compared to a typical patient.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, or of other Factor VIII products.
All patients were monitored for neutralizing antibodies (inhibitors) to Factor VIII in the clinical program. No patients developed neutralizing antibodies to Factor VIII.
During ALTUVIIIO clinical studies (median treatment duration 96.3 weeks), 4/276 (1.4%) of evaluable patients developed transient treatment emergent anti-drug antibodies.
No impact of ADAs on the FVIII activity levels or PK exposure parameters was observed.
No impact of ADAs with respect to bleeding episodes pharmacodynamic response, or safety was noted.
14. Clinical Studies
The safety, efficacy, and pharmacokinetics of ALTUVIIIO were evaluated in two multicenter, prospective, open-label clinical studies (one study in adults and adolescents ≥12 years of age and one pediatric study in children <12 years of age) in PTPs with severe hemophilia A (<1% endogenous Factor VIII activity or a documented genetic mutation consistent with severe hemophilia A).
All studies evaluated the efficacy of routine prophylaxis with a weekly dose of 50 IU/kg and determined hemostatic efficacy in the treatment of bleeding episodes and during perioperative management in patients undergoing major or minor surgical procedures.
The adult and adolescent study enrolled a total of 159 PTPs (158 male and 1 female patients) with severe hemophilia A. Patients were aged 12 to 72 years and included 25 adolescent patients aged 12 to 17 years. All 159 enrolled patients received at least one dose of ALTUVIIIO and were evaluable for efficacy. A total of 149 patients (93.7%) completed the study.
The pediatric study enrolled 74 male PTPs <12 years of age with severe hemophilia A (38 patients were 1 to 5 years of age and 36 were 6 to 11 years of age). Of the 74 enrolled patients, all received at least 1 dose of ALTUVIIIO. Seventy-two patients were evaluable for efficacy.
Routine Prophylaxis to Reduce Bleeding Episodes
Adult and Adolescent Study
The efficacy of weekly 50 IU/kg ALTUVIIIO as routine prophylaxis was evaluated as estimated by the mean annualized bleed rate (ABR) and by comparing the ABR during on-study prophylaxis vs. the ABR during pre-study FVIII prophylaxis. A total of 133 adults and adolescents, who were on pre-study FVIII prophylaxis, were assigned to receive ALTUVIIIO for routine prophylaxis at a dose of 50 IU/kg IV once weekly for 52 weeks (Arm A). An additional 26 patients, who were on pre-study episodic (on-demand) treatment with FVIII, received episodic (on-demand) treatment with ALTUVIIIO at doses of 50 IU/kg IV for 26 weeks, followed by routine prophylaxis at a dose of 50 IU/kg IV once weekly for 26 weeks (Arm B). Overall, 115 patients received at least a total number of 50 exposure days (EDs) in Arm A and 17 patients completed at least 25 EDs of routine prophylaxis in Arm B.
The ABR in patients evaluable for efficacy with at least 26 weeks of exposure are summarized in Table 6.
| Endpoint* | Arm A Prophylaxis† | Arm B On-demand‡ | Arm B Prophylaxis‡ |
|---|---|---|---|
| ABR = annualized bleed rate; CI = confidence interval; Q1 = 25th percentile, Q3 = 75th percentile. | |||
| N = 128 | N = 26 | N = 26 | |
| Treated bleeds | |||
| Mean ABR (95% CI)§ | 0.7 (0.5, 1.0) | 21.4 (18.8, 24.4) | 0.7 (0.3, 1.5) |
| Median ABR (Q1, Q3) | 0 (0, 1.0) | 21.1 (15.1, 27.1) | 0 (0, 0) |
| % patients with zero bleeds, n (%) | 82 (64.1) | 0 | 20 (76.9) |
| Treated spontaneous bleeds | |||
| Mean ABR (95% CI)§ | 0.3 (0.2, 0.4) | 15.8 (12.3, 20.4) | 0.4 (0.2, 1.2) |
| Median ABR (Q1, Q3) | 0 (0, 0) | 16.7 (8.6, 23.8) | 0 (0, 0) |
| % patients with zero bleeds, n (%) | 103 (80.5) | 1 (3.8) | 22 (84.6) |
| Treated joint bleeds | |||
| Mean ABR (95% CI)§ | 0.5 (0.4, 0.7) | 17.5 (14.9, 20.5) | 0.6 (0.3, 1.5) |
| Median ABR (Q1, Q3) | 0 (0, 1.0) | 18.4 (10.8, 23.9) | 0 (0, 0) |
| % patients with zero bleeds, n (%) | 92 (71.9) | 0 | 21 (80.8) |
| All Bleeds (treated and untreated)* | |||
| Mean ABR (95% CI)§ | 1.1 (0.8, 1.5) | 22.2 (19.4, 25.4) | 0.9 (0.4, 1.8) |
| Median ABR (Q1, Q3) | 0 (0, 1.2) | 21.1 (16.8, 27.1) | 0 (0. 1.9) |
| % patients with zero bleeds, n (%) | 71 (55.5) | 0 | 19 (73.1) |
An intra-patient comparison (N = 78) between mean ABR during on-study prophylaxis with ALTUVIIIO and that during pre-study FVIII prophylaxis yielded a 77% reduction in treated bleeds (95% CI: 58%, 87%).
All patients with target joints at baseline (defined as ≥3 spontaneous bleeding episodes in a major joint which occurred in a consecutive 6-month period) achieved resolution of all target joints (45/45, 100%) with 12 months of prophylactic treatment with ALTUVIIIO (defined as ≤2 bleeding episodes in the target joint in 12 months).
Pediatric Study
The efficacy of weekly 50 IU/kg ALTUVIIIO as routine prophylaxis in children <12 years was evaluated as estimated by the mean annualized bleed rate (ABR). A total of 74 children (38 children <6 years of age and 36 children 6 to <12 years of age) were enrolled to receive ALTUVIIIO for routine prophylaxis at a dose of 50 IU/kg IV once weekly for 52 weeks.
The ABR in patients evaluable for efficacy with at least 26 weeks of exposure are summarized in Table 7.
| Endpoint* | <6 years | 6 to <12 years | Overall |
|---|---|---|---|
| N = 37† | N = 35‡ | N = 72†‡ | |
| ABR = annualized bleed rate; CI = confidence interval; Q1 = 25th percentile, Q3 = 75th percentile. | |||
|
|||
| Treated bleeds | |||
| Mean ABR (95% CI)§ | 0.5 (0.3, 0.8) | 0.8 (0.4, 1.4) | 0.6 (0.4, 0.9) |
| Median ABR (Q1, Q3) | 0 (0, 1.0) | 0 (0, 1.1) | 0 (0, 1.0) |
| % patients with zero bleeds, n (%) | 23 (62.2) | 23 (65.7) | 46 (63.9) |
| Treated spontaneous bleeds | |||
| Mean ABR (95% CI)§ | 0.2 (0.1, 0.4) | 0.2 (0, 0.6) | 0.2 (0.1, 0.3) |
| Median ABR (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| % patients with zero bleeds, n (%) | 31 (83.8) | 32 (91.4) | 63 (87.5) |
| Treated joint bleeds | |||
| Mean ABR (95% CI)§ | 0.2 (0.1, 0.6) | 0.4 (0.2, 0.9) | 0.3 (0.2, 0.6) |
| Median ABR (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| % patients with zero bleeds, n (%) | 33 (89.2) | 27 (77.1) | 60 (83.3) |
| All Bleeds (treated and untreated)* | |||
| Mean ABR (95% CI)§ | 2.8 (1.4, 5.6) | 2.3 (1.3, 4.1) | 2.6 (1.6, 4.0) |
| Median ABR (Q1, Q3) | 0 (0, 2.0) | 1.0 (0, 2.9) | 0.5 (0, 2.1) |
| % patients with zero bleeds, n (%) | 20 (54.1) | 16 (45.7) | 36 (50.0) |
Efficacy in Control of Bleeding
In the adult and adolescent study, a total of 362 bleeding episodes were treated with ALTUVIIIO, most occurring during on-demand treatment in Arm B. Majority of bleeding episodes were localized in joints. Response to the first injection was assessed by patients at least 8 hours after treatment. A 4-point rating scale of excellent, good, moderate, and no response was used to assess response. Bleeding was resolved with a single 50 IU/kg injection of ALTUVIIIO in 96.7% of bleeding episodes. The median (Q1; Q3) total dose to treat a bleeding episode was 50.9 IU/kg (50.0; 51.9). Control of bleeding episodes was similar across the treatment arms.
The efficacy of ALTUVIIIO in control of bleeding in children <12 years of age was assessed in the pediatric study. A total of 43 bleeding episodes were treated with ALTUVIIIO. Bleeding was resolved with a single 50 IU/kg injection of ALTUVIIIO in 95.3% of bleeding episodes. The median (Q1; Q3) total dose to treat a bleeding episode was 52.6 IU/kg (50.0; 55.8).
Perioperative Management of Bleeding
Perioperative hemostasis was assessed in 14 major surgeries in 13 patients (10 adults and 3 children) across the adult and adolescent and pediatric clinical studies. Of the 14 major surgeries, 13 surgeries required a single pre-operative dose to maintain hemostasis during surgery; for 1 major surgery during routine prophylaxis no pre-operative loading dose was administered on the day of/or before surgery. The median pre-operative dose per surgery was 50 IU/kg (range 12.7 – 61.9).
The clinical evaluation of hemostatic response during major surgery was assessed using a 4-point scale of excellent, good, moderate, or poor/none. The hemostatic effect of ALTUVIIIO was rated as "excellent" in 14 of 14 surgeries (100%).
Types of major surgeries assessed include major orthopedic procedures such as arthroplasties (joint replacements of knee, hip, and elbow), joint revisions and ankle fusion. Other major surgeries included molar tooth extractions, dental restoration and tooth extraction, circumcision, and rhinoplasty/mentoplasty.
Perioperative hemostasis was assessed in 27 minor surgeries in 23 patients (12 adults and 11 adolescents and children). The hemostatic response was evaluated by the investigator/surgeon in 21 of these minor surgeries; an excellent response was reported in all (100%).
16. How is Altuviiio supplied
How Supplied
ALTUVIIIO is supplied in kits comprising a single-dose vial containing nominally, 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU) of Factor VIII potency, a prefilled syringe with 3 mL sterile water for injection, and a sterile vial adapter (reconstitution device). The actual amount of ALTUVIIIO in IU is stated on the label and carton of each vial.
Not made with natural rubber latex.
| Strength | Potency Color Code | Kit NDC Number |
|---|---|---|
| 250 IU | Yellow | 71104-978-01 |
| 500 IU | Red | 71104-979-01 |
| 750 IU | Garnet | 71104-980-01 |
| 1000 IU | Green | 71104-981-01 |
| 2000 IU | Royal Blue | 71104-982-01 |
| 3000 IU | Mist Grey | 71104-983-01 |
| 4000 IU | Orange | 71104-984-01 |
Not all pack sizes may be marketed.
Storage and Handling
Prior to reconstitution:
- Store ALTUVIIIO in the original package to protect the ALTUVIIIO vials from light.
- Store ALTUVIIIO in powder form at 2°C to 8°C (36°F to 46°F). Do not freeze to avoid damage to the prefilled diluent syringe.
- ALTUVIIIO may be stored at room temperature, not to exceed 30°C (86°F), for a single period of up to 6 months, within the expiration date printed on the label.
- If stored at room temperature, record the date that ALTUVIIIO is removed from refrigeration on the carton in the area provided. After storage at room temperature, do not return the product to the refrigerator.
- Do not use beyond the expiration date printed on the vial or 6 months after the date that was written on the carton, whichever is earlier.
After Reconstitution:
- The reconstituted product may be stored at room temperature, not to exceed 30°C (86°F), for up to 3 hours. Protect from direct sunlight. After reconstitution, if the product is not used within 3 hours, it must be discarded.
- Do not use ALTUVIIIO if the reconstituted solution is cloudy or has particulate matter.
- Discard any unused ALTUVIIIO.
17. Patient Counseling Information
Advise the patients to:
- Read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Call their healthcare provider or go to the emergency department right away if a hypersensitivity reaction occurs. Early signs of hypersensitivity reactions may include rash, hives, itching, facial swelling, tightness of the chest, and wheezing.
- Contact their healthcare provider or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to Factor VIII therapy because this may be a sign of inhibitor development.
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
©2025 Bioverativ Therapeutics Inc. All rights reserved.
For patent information: https://www.sanofi.us/en/products-and-resources/patents
ALTUVIIIO is a registered trademark of Bioverativ Therapeutics Inc.
Patient Information
ALTUVIIIO® (al too'vee oh)
[antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl]
for intravenous use after reconstitution only
Single-dose vial
Please read this Patient Information carefully before using ALTUVIIIO and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I need to know about ALTUVIIIO?
Do not attempt to give yourself an injection unless you have been taught how by your healthcare provider or hemophilia center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for injecting ALTUVIIIO so that your treatment will work best for you.
What is ALTUVIIIO?
ALTUVIIIO is an injectable medicine that is used to control and reduce the number of bleeding episodes in people with Hemophilia A (congenital Factor VIII deficiency).
Your healthcare provider may give you ALTUVIIIO when you have surgery.
Who should not use ALTUVIIIO?
You should not use ALTUVIIIO if you had an allergic reaction to it in the past.
What should I tell my healthcare provider before using ALTUVIIIO?
Talk to your healthcare provider about:
- Any medical problems that you have or had.
- All prescription and non-prescription medicines that you take, including over-the-counter medicines, supplements or herbal medicines.
- Pregnancy or if you are planning to become pregnant. It is not known if ALTUVIIIO may harm your unborn baby.
- Breastfeeding. It is not known if ALTUVIIIO passes into the milk and if it can harm your baby.
How should I use ALTUVIIIO?
You get ALTUVIIIO as an injection into your vein. Your healthcare provider will instruct you on how to do injections on your own and may watch you give yourself the first dose of ALTUVIIIO.
Contact your healthcare provider right away if bleeding is not controlled after using ALTUVIIIO.
What are the possible side effects of ALTUVIIIO?
You can have an allergic reaction to ALTUVIIIO. Call your healthcare provider or emergency department right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash or hives.
Your body can also make antibodies called "inhibitors" against ALTUVIIIO. This can stop ALTUVIIIO from working properly. Your healthcare provider may give you blood tests to check for inhibitors.
The common side effects of ALTUVIIIO are headache and joint pain.
These are not the only possible side effects of ALTUVIIIO. Tell your healthcare provider about any side effect that bothers you or does not go away.
What are the ALTUVIIIO dosage strengths?
ALTUVIIIO comes in seven different dosage strengths with 3 mL sterile water for injection (sWFI). The actual number of international units (IU) of Factor VIII activity in the vial will be imprinted on the label and on the box. The seven different strengths are as follows:
| Strength | Cap Color |
|---|---|
| 250 IU | Yellow |
| 500 IU | Red |
| 750 IU | Garnet |
| 1000 IU | Green |
| 2000 IU | Royal Blue |
| 3000 IU | Mist Grey |
| 4000 IU | Orange |
Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
How should I store ALTUVIIIO?
- Keep ALTUVIIIO in its original package.
- Protect it from light.
- Do not freeze.
- Store refrigerated 2°C to 8°C (36°F to 46°F) up to 48 months or at room temperature [not to exceed 30°C (86°F)], for a single period up to 6 months. Do not use ALTUVIIIO after the expiration date printed on the label and carton of each vial.
- When storing at room temperature:
- –
- Note on the carton the date on which the product is removed from refrigeration.
- –
- Use the product before the end of this 6-month period or discard it.
- –
- Do not return the product to the refrigerator.
After mixing with the diluent:
- Do not use ALTUVIIIO if the mixed solution is not clear and colorless to slightly yellowish.
- Use mixed product as soon as possible.
- You may store mixed ALTUVIIIO at room temperature, not to exceed 30°C (86°F), for up to 3 hours. Protect the mixed ALTUVIIIO from direct sunlight. Discard any mixed ALTUVIIIO not used within 3 hours.
What else should I know about ALTUVIIIO?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use ALTUVIIIO for a condition for which it was not prescribed. Do not share ALTUVIIIO with other people, even if they have the same symptoms that you have.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
©2024 Bioverativ Therapeutics Inc. All rights reserved.
ALTUVIIIO is a registered trademark of Bioverativ Therapeutics Inc.
Revised: SEP 2024
| INSTRUCTIONS FOR USE ALTUVIIIO® (al too'vee oh) [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] for intravenous use after reconstitution only Single-dose vial |
|
| Read the Instructions for Use before you start using ALTUVIIIO and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. Your healthcare provider should show you or your caregiver how to mix (reconstitute) and give ALTUVIIIO the first time ALTUVIIIO is used. Any further questions? Ask your healthcare provider or call 1-800-633-1610. |
|
| Important Information You Need to Know Before Injecting ALTUVIIIO It is important that you do not try to inject ALTUVIIIO unless you have received training from a healthcare provider.
|
|
Storing ALTUVIIIO
Storing of Mixed (Reconstituted) ALTUVIIIO
|
|
| Preparing to Inject ALTUVIIIO Mixing (Reconstitution) |
|
| Step 1: | |
 | Look at the ALTUVIIIO kit:
|
| Step 2: | |
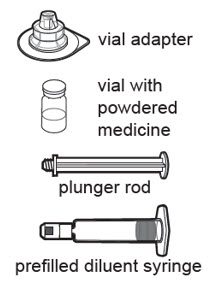  | Wash your hands with soap and water. Find a clean, flat work surface. Remove the supplies from the carton:
Also ensure you have the following supplies (not included in the carton):
|
| Step 3: | |
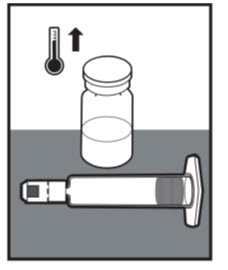 | Allow the ALTUVIIIO vial and the prefilled diluent syringe to come to room temperature before use.
|
| Step 4: | |
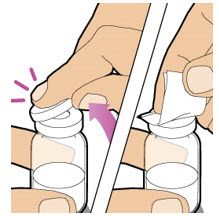 | Remove the plastic cap from the ALTUVIIIO vial. Wipe the rubber stopper of the vial with an alcohol wipe and allow it to dry.
|
| Step 5: | |
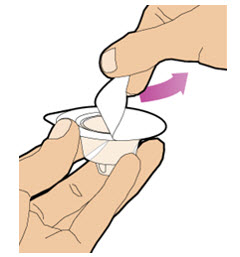 | Completely remove the backing from the vial adapter package by peeling back the lid.
|
| Step 6: | |
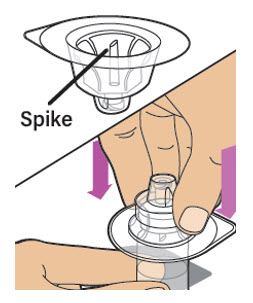 | Keep the vial on a flat surface. Hold the vial with one hand and using the other hand, place the vial adapter in its package over the vial. Note: The spike should be placed directly above the center of the rubber stopper.
|
| Step 7: | |
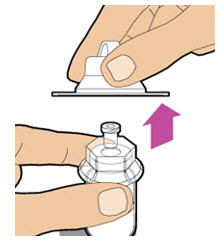 | Lift the package cover away from the vial adapter and throw away the cover. |
| Step 8: | |
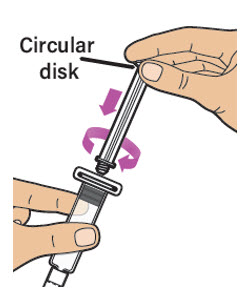 | Note: Only use the prefilled diluent syringe provided to mix (reconstitute) the powdered medicine.
|
| Step 9: | |
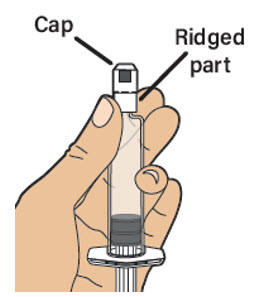 | With one hand, hold the prefilled diluent syringe directly under the cap with the cap pointing up. Note: Make sure you are holding the prefilled diluent syringe by the ridged part directly under the cap.
|
| Step 10: | |
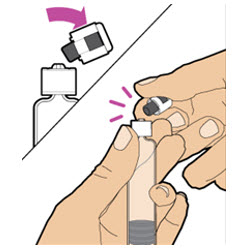 | With your other hand, grasp the cap and bend it at a 90-degree angle until it snaps off. Note: After the cap snaps off, you will see the glass tip of the prefilled diluent syringe.
|
| Step 11: | |
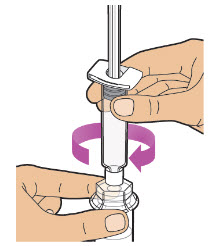 | Note: Be sure the vial is sitting on a flat surface.
|
| Step 12: | |
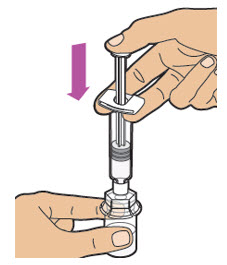 | Slowly push down on the plunger rod to inject all of the liquid (diluent) from the prefilled diluent syringe into the vial. Note: The plunger rod may rise slightly afterward. This is normal. |
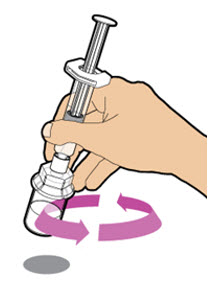 | With the prefilled diluent syringe still connected to the adapter, gently swirl the vial until the powder is completely dissolved. Check the solution through the vial to make sure the powder is fully dissolved. Note: The solution should look clear and colorless to slightly yellowish.
|
| Step 14: | |
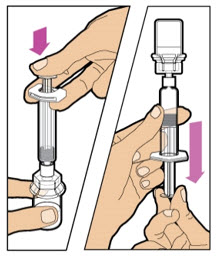 | Make sure the plunger rod is pressed all the way down and the syringe is firmly attached to the vial adapter. Turn the vial upside-down. Slowly pull down on the plunger rod to draw all the solution from the vial into the syringe. Note: Be careful not to pull the plunger rod completely out of the syringe. |
| Step 15: | |
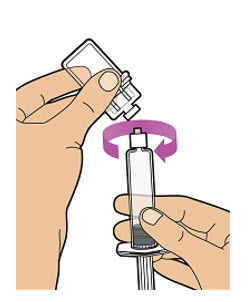 | Gently unscrew the syringe from the vial adapter by turning it to the right. Throw away (dispose of) the vial with the adapter still attached (See Step 23). If you are not ready to inject, put the syringe cap carefully back onto the syringe tip.
Note: Mixed (reconstituted) ALTUVIIIO should be given within 3 hours after mixing. Protect from direct sunlight. Do not refrigerate after reconstitution. |
| Injecting ALTUVIIIO | |
| ALTUVIIIO is given in the vein (intravenous injection) after mixing (reconstitution) of the powdered medicine with the diluent. These are very generic instructions so your healthcare provider should teach you how to inject ALTUVIIIO. After you have been taught to self-inject, you can follow these instructions.
|
|
| Step 16: | |
 | Using aseptic technique (clean and germ free), attach the syringe to the connector end of the infusion set tubing by turning it to the right until securely attached.
Note: Ask your healthcare provider which infusion set can be used with ALTUVIIIO. |
| Step 17: | |
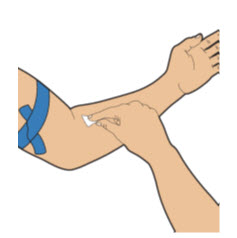 | Apply a tourniquet to the upper arm. Then, using a new alcohol wipe, clean the skin where you will insert the needle and wait for it to dry. |
| Step 18: | |
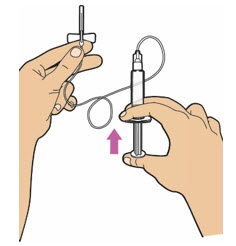 | Prime the syringe and the tubing. Push the plunger rod until all air is removed from the syringe and ALTUVIIIO has filled the infusion set needle.
|
| Step 19: | |
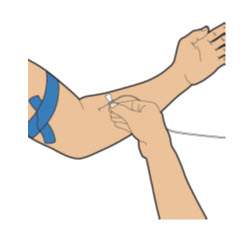 | Remove the protective needle cover from the infusion set needle and throw away (discard it).
|
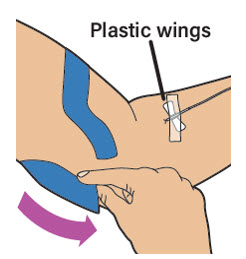 | Use tape to secure the plastic wings of the needle in place at the injection site if needed. Remove the tourniquet. Note: Always make sure you have correctly inserted the needle into a vein when you perform an intravenous injection. |
| Step 21: | |
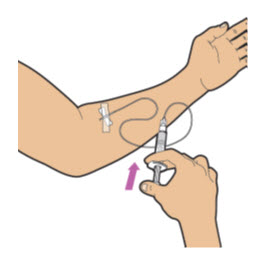 | Slowly push the plunger rod on the syringe all the way down to give ALTUVIIIO. Your healthcare provider will provide your rate of administration based on your comfort level and the minimum injection time recommendation per vial. Note: A small amount of medicine will be left in the infusion set after injection. This is normal. |
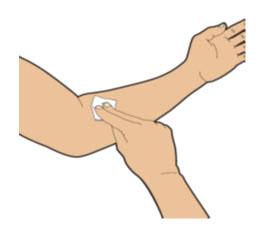 | After delivering ALTUVIIIO, remove the tape and the needle from the vein. Use a cotton ball or gauze pad to put pressure on the injection site for several minutes to stop any bleeding. Note: You may apply an adhesive bandage if needed. |
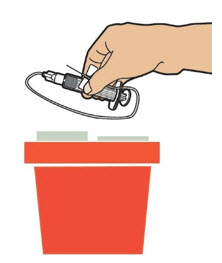 | Put the infusion set and the syringe in FDA-cleared sharps disposal container right away after use. Dispose of all unused solution according to your local regulations. Dispose of all empty vial(s), and other used medical supplies in your household trash.
|
| Disposing of ALTUVIIIO
Throw away (dispose of) the infusion set and the syringe in FDA-cleared sharps disposal container right away after use. If you do not have FDA-cleared sharps disposal container you may use a household container that is:
When your FDA-cleared sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of FDA-cleared sharps disposal container. There may be state or local laws about how you should throw away used ALTUVIIIO supplies.
Keep your FDA-cleared sharps disposal container out of the reach of children. |
|
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
©2024 Bioverativ Therapeutics Inc. All rights reserved.
For more information go to www.ALTUVIIIO.com or call 1-800-633-1610
Revised: SEP 2024
PRINCIPAL DISPLAY PANEL - 250 IU Kit Carton
250 IU Nominal
NDC 71104-978-01
ALTUVIIIO™
[Antihemophilic Factor (Recombinant),
Fc-VWF-XTEN Fusion Protein-ehtl]
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
intravenous injection - (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
plunger rod - (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
www.ALTUVIIIO.com
1-800-633-1610
Diluent origin Germany
Plunger Rod origin France
Vial Adapter origin Israel
sanofi
PRINCIPAL DISPLAY PANEL - 500 IU Kit Carton
500 IU Nominal
NDC 71104-979-01
ALTUVIIIO™
[Antihemophilic Factor (Recombinant),
Fc-VWF-XTEN Fusion Protein-ehtl]
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
intravenous injection - (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
plunger rod - (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
www.ALTUVIIIO.com
1-800-633-1610
Diluent origin Germany
Plunger Rod origin France
Vial Adapter origin Israel
sanofi
PRINCIPAL DISPLAY PANEL - 1000 IU Kit Carton
1000 IU Nominal
NDC 71104-981-01
ALTUVIIIO™
[Antihemophilic Factor (Recombinant),
Fc-VWF-XTEN Fusion Protein-ehtl]
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
intravenous injection - (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
plunger rod - (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
www.ALTUVIIIO.com
1-800-633-1610
Diluent origin Germany
Plunger Rod origin France
Vial Adapter origin Israel
sanofi
PRINCIPAL DISPLAY PANEL - 2000 IU Kit Carton
2000 IU Nominal
NDC 71104-982-01
ALTUVIIIO™
[Antihemophilic Factor (Recombinant),
Fc-VWF-XTEN Fusion Protein-ehtl]
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
intravenous injection - (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
plunger rod - (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
www.ALTUVIIIO.com
1-800-633-1610
Diluent origin Germany
Plunger Rod origin France
Vial Adapter origin Israel
sanofi
PRINCIPAL DISPLAY PANEL - 3000 IU Kit Carton
3000 IU Nominal
NDC 71104-983-01
ALTUVIIIO™
[Antihemophilic Factor (Recombinant),
Fc-VWF-XTEN Fusion Protein-ehtl]
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
intravenous injection - (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
plunger rod - (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
www.ALTUVIIIO.com
1-800-633-1610
Diluent origin Germany
Plunger Rod origin France
Vial Adapter origin Israel
sanofi

PRINCIPAL DISPLAY PANEL - 4000 IU Kit Carton
4000 IU Nominal
NDC 71104-984-01
ALTUVIIIO™
[Antihemophilic Factor (Recombinant),
Fc-VWF-XTEN Fusion Protein-ehtl]
For Intravenous Use
This package contains:
- (1) Single-dose vial containing lyophilized powder for reconstitution for
intravenous injection - (1) Prefilled diluent syringe containing 3 mL sterile water for injection (sWFI) with
plunger rod - (1) Sterile vial adapter reconstitution device
- (1) Package Insert
Rx Only
Manufactured by:
Bioverativ Therapeutics Inc.
Waltham, MA 02451
A SANOFI COMPANY
US License Number 2078
www.ALTUVIIIO.com
1-800-633-1610
Diluent origin Germany
Plunger Rod origin France
Vial Adapter origin Israel
sanofi

| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bioverativ Therapeutics Inc. (070517011) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PPD Development Ireland Ltd. | 985036175 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc | 069777290 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biogen U.S. Corporation | 078734950 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , MANUFACTURE(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Rechon Life Science AB | 775207769 | PACK(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , LABEL(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | PACK(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , LABEL(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma Fertigung GmbH & Co. KG (Langenargen Eisenbahnstrasse) | 344217323 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , MANUFACTURE(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Schuetzenstrasse) | 316126754 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Mooswiesen) | 312670654 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , PACK(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Ireland Limited | 985127419 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , MANUFACTURE(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biogen MA Inc. | 841087823 | API MANUFACTURE(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
More about Altuviiio (antihemophilic factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
Patient resources
Professional resources
Other brands
Advate, Xyntha, Esperoct, Recombinate, ... +12 more






 After cleaning,
After cleaning, 











