Nuwiq Dosage
Generic name: Simoctocog Alfa 4000[iU] in 2.5mL;
Dosage form: injection
Drug class: Miscellaneous coagulation modifiers
Medically reviewed by Drugs.com. Last updated on Dec 26, 2024.
For intravenous use after reconstitution
Dose
- Each vial of NUWIQ is labeled with the actual Factor VIII potency expressed in international units (IU). One IU of Factor VIII activity is defined by the quantity of Factor VIII in one mL of normal human pooled plasma. Calculation of the required dose of Factor VIII is based on the empirical finding that 1 IU Factor VIII per kg body weight raises the plasma Factor VIII activity by approximately 2% of normal activity or 2 IU/dL when assessed using the one stage clotting assay. Use the following formulae to determine the required dose:
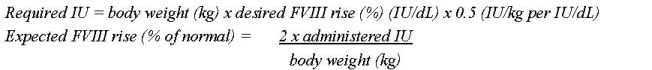
- Dose and duration of therapy depend on the severity of the Factor VIII deficiency, the location and extent of the bleeding, FVIII level, and the patient’s clinical condition.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing NUWIQ for the on-demand treatment and control of bleeding episodes is provided in Table 1 . Selected dosing regimen should maintain plasma Factor VIII activity levels at or above the plasma levels (in % of normal or in IU/dL) outlined in the table.
Table 1: Dosing for Treatment and Control of Bleeding Episodes
| Type of Bleeding Episodes | Required peak post-infusion Factor VIII activity (% of normal or IU/dL) |
Frequency of Dosing (hours) | Duration of Therapy (days) |
| Minor: Superficial muscle or soft tissue and oral bleeds |
20–40 | 12-24 | At least 1 day, until the bleeding episode is resolved. |
| Moderate to Major: Hemorrhage into muscles, into oral cavity, hemarthrosis, known trauma |
30–60 | 12-24 | For 3–4 days or more until bleeding episode is resolved. |
| Life-threatening: Intracranial, intra-abdominal, gastro-intestinal or intrathoracic bleeds, central nervous system bleeds, bleeding in retropharyngeal spaces or iliopsoas sheath, eyes/retina, fractures or head trauma | 60–100 | 8-24 | Until bleeding risk is resolved. |
Perioperative Management
A guide for dosing NUWIQ during surgery (perioperative management) is provided in Table 2 . Dosing should aim at maintaining a plasma Factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in the table.
Table 2: Dosing for Perioperative Management
| Type of Surgery | Required post-infusion Factor VIII activity (% of normal or IU/dL) |
Frequency of Doses (hours) | Duration of Therapy (days) |
| Minor including tooth extraction |
30–60 (pre- and post-operative) |
24 | At least 1 day, until healing is achieved. |
| Major Intracranial, intra-abdominal, or joint-replacement therapy |
80–100 (pre- and post-operative) |
8-24 | Until adequate wound healing, then continue therapy for at least another 7 days to maintain a Factor VIII activity of 30% to 60% (IU/dL). |
Routine Prophylaxis
A guide for dosing NUWIQ for routine prophylaxis to reduce the frequency of bleeding is provided in Table 3 . Exact dosing should be defined by the patient’s clinical status and response.
Table 3: Dosing for Routine Prophylaxis
| Patients | Dose (IU/kg) | Frequency of infusions |
| Adults and adolescents 12 - 17 yrs | 30 - 40 | Every other day |
| Children 2 - 11 yrs | 30 - 50 | Every other day or three times per week |
| Children < 2 yrs | 20 - 50 | Once per week to every other day |
A regimen may be further individually adjusted to less or more frequent dosing at the discretion of the treating physician.
Preparation and Reconstitution
NUWIQ package contents:
- single-dose vial of NUWIQ concentrate
- pre-filled syringe containing 2.5 mL Sterile Water for Injection
- vial adapter
- butterfly needle
- two alcohol swabs.
- Always work on a clean surface and wash your hands before performing the procedure.
- Allow the vial of NUWIQ and the pre-filled syringe to come to room temperature.
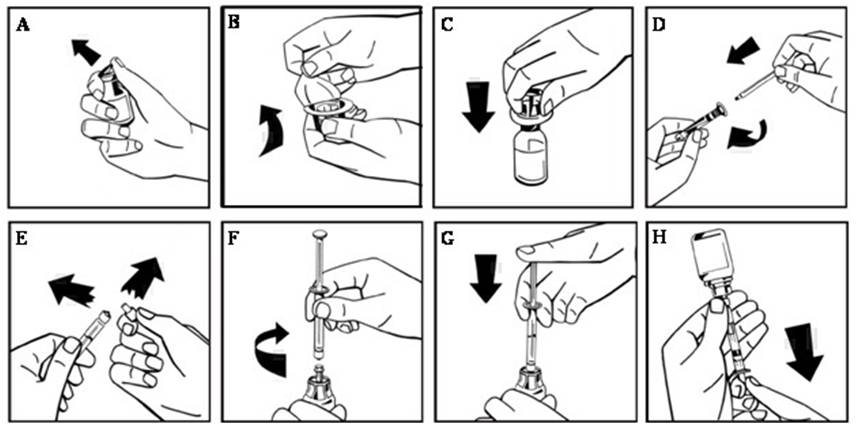
- Remove the plastic flip-top cap from the NUWIQ vial to expose the rubber stopper. (Figure A).
- Wipe the top of the vial with an alcohol swab and allow the rubber stopper of the vial to dry.
- Peel back the paper cover from the vial adapter package revealing the adapter spike without removing the adapter from the package (Figure B).
- With the concentrate vial on an even surface, insert the adapter spike into the rubber stopper. The adapter snaps to the vial when done (Figure C).
- Peel back the paper cover from the pre-filled syringe package. Connect plunger rod attaching the threaded end of the plunger rod to the solvent syringe, turning clockwise until a slight resistance is felt (Figure D). Avoid contact with shaft.
- Break off the tamper-proof plastic tip from the syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip (Figure E).
- Remove the adapter packaging and connect the syringe to the vial adapter by turning clockwise until resistance is felt (Figure F).
- Slowly inject all liquid from syringe into the concentrate vial (Figure G).
- Without removing the syringe, dissolve the concentrate powder in the vial by gently moving or swirling a few times. DO NOT SHAKE. Wait until all the powder dissolves completely.
- Inspect the final solution for particles. The solution should be clear, colorless, and free from visible particles. Do not use if solution is cloudy or has particulate matter.
- Turn the vial and syringe upside down (still attached).
- Slowly withdraw the solution into the syringe. Make sure that all liquid is transferred to the syringe (Figure H).
- Detach the filled syringe from the vial adapter by turning counter clockwise.
Do not refrigerate the solution after reconstitution. Use the solution within 3 hours after reconstitution. If solution is not used within this time period, close the filled syringe with the tamper-proof plastic tip, and discard.
Administration
For intravenous use after reconstitution only
- Inspect the reconstituted NUWIQ solution for visible particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not administer NUWIQ in the same tubing or container as other medications.
- Clean the chosen injection site with an alcohol swab.
- Attach the provided infusion set to the syringe. Insert the needle of the infusion set into the chosen vein.
- Perform intravenous bolus infusion. The rate of administration should be determined by patient’s comfort level, at a maximum rate of 4 mL per minute.
- After infusing NUWIQ, remove and properly discard the infusion set. After the infusion, remove the peel-off label containing the batch number from the factor concentrate vial and place it in the log book for record keeping.
More about Nuwiq (antihemophilic factor)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español
Patient resources
Other brands
Altuviiio, Recombinate, Advate, Jivi, ... +14 more
Professional resources
Other brands
Altuviiio, Recombinate, Advate, Jivi, ... +12 more
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.

