Apremilast
Generic name: apremilast [ a-PRE-mi-last ]
Brand name: Otezla
Dosage form: oral tablet (10 mg, 20 mg, 30 mg)
Drug class: Antirheumatics
What is apremilast?
Apremilast (Otezla) is used to treat plaque psoriasis, psoriatic arthritis, and Behçet's disease in specific patients. Apremilast tablets help improve plaque psoriasis for clearer skin and reduce inflammation and symptoms such as pain, redness, and swelling in psoriatic arthritis and Behcet's disease.
Apremilast is a PDE4 inhibitor that works by increasing the level of cAMP (cyclic adenosine monophosphate), a messenger involved in cell signaling in our bodies. Apremilast is not a biologic agent.
Apremilast, under the brand name Otezla, received FDA approval on March 21, 2014, for psoriatic arthritis. Since then, it has also been FDA-approved to treat types of plaque psoriasis and Behcet’s disease in specific patients. It is not available as a generic medicine.
What is Apremilast used for?
- Plaque Psoriasis: Adult patients with plaque psoriasis who require systemic therapy or light treatment (phototherapy). Pediatric patients 6 years of age and older and weighing at least 20 kg with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
- Psoriatic Arthritis: Treatment for adult patients with active psoriatic arthritis.
- Oral Ulcers Associated with Behçet's Disease: Treatment for adult patients with oral ulcers associated with Behçet's Disease (a disease causing inflammation in blood vessels).
Warnings
Some people have thoughts about suicide while taking apremilast. Stay alert to changes in your mood or symptoms. Report any new or worsening symptoms to your doctor.
Before taking this medicine
You should not use apremilast if you are allergic to it.
To make sure this medicine is safe for you, tell your doctor if you have ever had:
-
kidney disease; or
-
depression or suicidal thoughts or actions.
Some people have thoughts about suicide while taking apremilast. Your doctor will need to check your progress at regular visits. Your family or other caregivers should also be alert to changes in your mood or symptoms.
Apremilast may harm an unborn baby. Use effective birth control to prevent pregnancy, and tell your doctor if you become pregnant.
If you are pregnant, your name may be listed on a pregnancy registry to track the effects of apremilast on the baby.
It may not be safe to breastfeed while using this medicine. Ask your doctor about any risk.
Apremilast is not approved for use by anyone younger than 18 years old.
How should I take apremilast?
Take apremilast exactly as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets. Your doctor may occasionally change your dose.
You may take apremilast with or without food.
Swallow the tablet whole, and do not crush, chew, or break it.
Keep track of your body weight while you are taking this medicine, and tell your doctor about any major weight loss.
Store at room temperature, away from moisture and heat.
Dosing information
Usual Adult Apremilast Dose for Psoriatic Arthritis, Plaque Psoriasis, and Behcet’s disease
Adults Starting dose:
- Day 1: 10 mg once a day in the morning
- Day 2: 10 mg twice a day (morning and evening)
- Day 3: 10 mg in the morning and 20 mg in the evening
- Day 4: 20 mg twice a day (morning and evening)
- Day 5: 20 mg in the morning and 30 mg in the evening, then maintenance dose.
Maintenance dose:
- 30 mg orally twice a day (morning and evening)
Usual Apremilast Dose Pediatric Patients with Moderate to Severe Plaque Psoriasis who are 6 Years of Age and Older and Weighing at Least 20kg.
Pediatric Patients 20kg to less than 50 kg:
- Day 1: 10 mg once a day in the morning
- Day 2: 10 mg twice a day (morning and evening)
- Day 3: 10 mg in the morning and 20 mg in the evening
- Day 4: 20 mg twice a day (morning and evening), then maintenance dose.
Maintenance dose:
- 20 mg twice a day (morning and evening)
Pediatric Patients 50 kg or more:
- Day 1: 10 mg once a day in the morning
- Day 2: 10 mg twice a day (morning and evening)
- Day 3: 10 mg in the morning and 20 mg in the evening
- Day 4: 20 mg twice a day (morning and evening)
- Day 5: 20 mg in the morning and 30 mg in the evening then maintenance dose.
Maintenance dose:
- 30 mg orally twice a day (morning and evening)
General Dosing Comments
Keep track of your body weight while you are taking this medicine, and tell your doctor about any major weight loss.
Dosage adjustments are required in renal impairment.
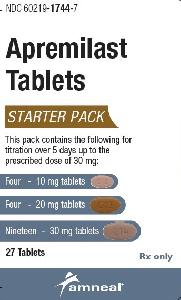
Apremilast usually begins at a low dose that is increased over 5 days to the maintenance dose, this is to reduce gastrointestinal symptoms that may happen when you first start taking this medicine. You may be given your medicine in a starter pack containing tablets that have different strengths; it is important to follow your doctor's instructions carefully.
What happens if I miss a dose?
Take the medicine as soon as you can, but skip the missed dose if it is almost time for your next dose. Do not take two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222.
What should I avoid while taking apremilast?
Follow your doctor's instructions about any restrictions on food, beverages, or activity.
Apremilast side effects
Common apremilast side effects may include:
- nausea, diarrhea;
- headache; or
- cold symptoms such as stuffy nose, sneezing, sore throat.
Serious apremilast side effects
Get emergency medical help if you have signs of an allergic reaction to this medicine: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Apremilast may cause other serious side effects. Call your doctor at once if you have:
- severe diarrhea, nausea, and vomiting;
- unexplained weight loss, or if you lose a lot of weight;
- mood changes, new or worsening depression; or
- thoughts of suicide or hurting yourself.
This is not a complete list of side effects, and others may occur. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
What other drugs will affect apremilast?
Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.
Other drugs may interact with apremilast, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.
Popular FAQ
Sotyktu and Otezla are both oral prescription medicines used to treat moderate-to-severe plaque psoriasis, but they work in different ways. Sotyktu blocks a protein called TYK2 (tyrosine kinase 2) to help lower inflammation and improve the severity and number of psoriasis lesions. Otezla works by blocking the phosphodiesterase type 4 (PDE4) enzyme to lower inflammation. Continue reading
Otezla (apremilast) is approved to treat plaque psoriasis in adults and children 6 years and older (and weighing at least 20 kg), as well as psoriatic arthritis and Behçet’s Disease in adults. Patients may start to feel an improvement within the first few weeks, but symptoms can further improve over 12 to 16 weeks. Continue reading
In your body, Otezla blocks the action of the PDE4 enzyme to help control inflammatory symptoms such as pain, redness and swelling in conditions like psoriasis and psoriatic arthritis. Continue reading
Otezla is made by Amgen Inc, located in Thousand Oaks, California. Otezla is expensive because it is the original, brand name drug and Otezla's manufacturer sponsored the clinical development. The lower-cost generic option called apremilast was approved by the FDA in February 2021, but may not yet be on the market. Continue reading
More FAQ
References
More about apremilast
- Check interactions
- Compare alternatives
- Reviews (461)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: antirheumatics
- Breastfeeding
- En español
Patient resources
Other brands
Professional resources
Other brands
Related treatment guides
Further information
Remember, keep this and all other medicines out of the reach of children, never share your medicines with others, and use apremilast only for the indication prescribed.
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Copyright 1996-2024 Cerner Multum, Inc. Version: 2.01.