FDA-Approved Weight Loss Drugs: Can They Help You?
The latest type 2 diabetes and weight loss medicines like Zepbound, Mounjaro, Rybelsus, Wegovy, and Ozempic have led to significant blood sugar control and weight loss in clinical studies. But in the U.S., these newer drugs are often not covered by insurance due to high cost and lack of available generics. What options are available that can help you on your weight-loss journey?
Here, we'll review some of the newer and older weight loss treatments and look at their advantages and drawbacks. While medicines can help with weight loss maintenance, diet and exercise are also used to lead to sustainable weight loss maintenance and should be added to your program.
Why is weight loss so important?
According to the U.S. Centers for Disease Control and Prevention (CDC), there has been a dramatic increase in obesity in the last 20 years. Obesity affects 4 out of every 10 adults and 2 out of every 10 children in the U.S., and accounts for close to $173 billion in health care costs every year.
These numbers are especially astounding because obesity and obesity-related conditions account for some of the leading causes of preventable deaths in the U.S. Obesity-related conditions include:
- High blood pressure
- High cholesterol and heart disease
- Stroke
- Type 2 diabetes
- Cancer types like endometrial, breast, colon, kidney, gallbladder, and liver
In addition, being overweight or obese is a recognized risk factor for many other major health problems including osteoarthritis, sleep apnea and breathing problems, mental health issues like depression, and gallbladder disease.
Weight loss drugs approved by the FDA since 2012 include:
- Zepbound (tirzepatide)
- Wegovy (semaglutide)
- Saxenda (liraglutide)
- Contrave (bupropion and naltrexone)
- Qsymia (phentermine and topiramate)
The generic liraglutide option for Victoza was approved by the FDA on December 23, 2024. The generic is only approved to be used to improve blood sugar control in type 2 diabetes (as an adjunct to diet and exercise), but it may also lead to some weight loss.
In December 2024 Zepbound was approved to treat moderate-to-severe obstructive sleep apnea (OSA) in adults with obesity. This was the first FDA-approved prescription treatment for this use.
Be sure to talk to your doctor for individual advice before starting any weight loss program, learn about drug side effects, and realize it takes time and discipline to achieve sustained weight loss. Learn more about exercise and food choices, too. You may also benefit from meeting with a registered dietician.
Related: How do Ozempic, Mounjaro, Wegovy & Zepbound compare for weight loss?
What is a BMI?
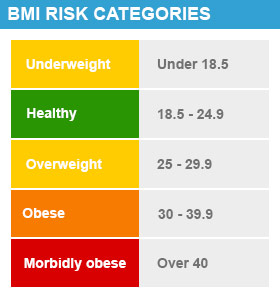
Your healthcare provider may calculate your body mass index (BMI) to better understand your weight risk category. It's important to understand how this is calculated so you can discuss it with your provider.
- BMI is a calculation of your weight in relation to your height that defines your health risk. BMI is calculated by dividing weight (in kg) by height (in meters) squared.
- Obesity is defined as a BMI over 30 kilograms/meter squared (kg/m2).
- A BMI of 25 to 30 is considered overweight. A BMI of 18.5 to 24.9 is considered healthy.
GLP-1 / GIP weight loss medicines
In general, you should consider weight loss medicines if you are obese, or overweight with weight-related conditions like type 2 diabetes, heart disease, high blood pressure, high cholesterol or sleep apnea. A healthy diet and regular exercise are part of the weight-loss regimen that should be continued even if weight loss medicines are stopped.
Weight loss agents are now available on the U.S. market that can help patients lose 20% or more of their weight when combined with diet and exercise. These agents work like natural hormones in the body, called glucose-dependent insulinotropic polypeptide (GIP) and / or glucagon-like peptide-1 (GLP-1) receptor agonists, which help control appetite, blood sugar levels, and digestion. They reduce your appetite and make you feel full more quickly and for a longer time period, so you eat less and lose weight.
Common side effects with GLP-1 and GLP-1 / GIP agents include stomach effects like nausea, vomiting, diarrhea, stomach area (abdominal) pain and constipation. These side effects tend to subside in most people after a few weeks and when you have reached your maintenance dose.
Also let your healthcare provider know if you have a procedure or surgery scheduled that will required sedation or general anesthesia.
- Rarely, GLP-1 and GLP-1 / GIP agonists can increase the risk of food or liquid getting into your lungs (pulmonary aspiration) during deep sedation or general anesthesia during procedures or surgery.
- Lung aspiration may occur because GLP-1 type drugs slow down stomach emptying. Even if you fast (avoid eating / drinking) for a period of time as directed before surgery, you may still have food or liquid left in your stomach, which could be inhaled into your lungs (aspirated) while under sedation or general anesthesia.
Tell all your healthcare providers that you are taking a weight loss drug before you are scheduled to have surgery or other procedures.
Related Questions
- Can Ozempic and Wegovy cause blindness?
- Does Rybelsus help with weight loss?
- What foods should I avoid when taking Rybelsus?
Zepbound vs. Wegovy
Zepbound (tirzepatide) or Wegovy (semaglutide) are given as a subcutaneous injection (under the skin) once a week, with or without meals. Injections come as a pre-filled, single-dose pen for injection. You or a caregiver can learn to give this injection at home. Medication is used in addition to diet and increased physical activity.
Zepbound, a GIP and GLP-1 receptor agonist from Eli Lilly, is used in combination with diet and increased physical activity, for chronic weight management in:
- adults with obesity
- adults with overweight and at least one weight-related medical condition (for example: high blood pressure, high cholesterol, type 2 diabetes, obstructive sleep apnea or heart disease).
Zepbound is a once-weekly injection given subcutaneously (under the skin) with a pre-filled single-dose pen. It contains tirzepatide, the same active ingredient that's in Lilly's Mounjaro, which is approved for type 2 diabetes (but also helps with weight loss).
- In the 72-week long SURMONT-1 study with over 2,500 adults, those using the highest Zepbound 15 mg dose lost an average of 21% of their body weight (21.8 kg or 48 lb). With the lower 5 mg dose, people lost an average of 15% of their body weight (15.5 kg or 34 lb). Overall, people taking a placebo (inactive) agent lost an average of 3% of their weight, or 3.2 kg (7 lb).
- The percent of patients that lost 5% or more of their body weight ranged from 85% to 91%, based on which dose they were prescribed.
- After 72 weeks of treatment, people who used Zepbound saw a statistically significant reduction in their body weight (compared with a placebo).
Zepbound is not approved for use in children under 18 years of age, and it is not known if use in this age group is safe or effective. In addition, Zepbound is not yet labeled to help reduce the risk of major adverse cardiovascular events (like heart-related death, heart attack, or a stroke).
Related: How does Zepbound help treat sleep apnea?
Wegovy, a GLP-1 receptor agonist injection from Novo Nordisk, is approved by the FDA for long-term weight management (in addition to diet and exercise) in:
- Adults and children aged 12 years and older with obesity
- Adults with overweight in the presence of at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol)
In March 2024 Wegovy was also approved to reduce the risk of major adverse cardiovascular events (like heart-related death, heart attack or a stroke) in adults with established heart disease and either obesity or overweight.
Wegovy contains semaglutide, the same active ingredient found in Ozempic (that is approved for type 2 diabetes, and for kidney and heart protection in certain patient groups). Ozempic can lead to significant weight loss too, even though it's not approved for this use.
In clinical studies, patients taking Wegovy (semaglutide) achieved an average weight loss of 14.9% of body weight at 68-weeks vs. 2.4% for placebo. In addition, 83.5% of patients achieved 5% or more body weight reduction in the Wegovy group compared to 31.1% for those taking a placebo (an inactive agent).
Weight loss with Wegovy may begin within the first few weeks of treatment, but the full effects of treatment may not be seen for at least several months.
Ozempic, Rybelsus and Mounjaro
Other GLP-1 prescription drugs, like Ozempic (a subcutaneous injection) or Rybelsus (oral tablets), also contain semaglutide and can lead to weight loss, but are approved to treat type 2 diabetes, together with diet and exercise (not weight loss) at this time.
- In two studies, patients using either the Ozempic 0.5 mg dose, 1 mg or 2 mg dose lost on average 8 lb (3.6 kg) to 14 lb (6.3 kg). Those taking a placebo (an inactive treatment) lost an average of 3 lb (1.4 kg).
- In Rybelsus studies, participants with type 2 diabetes taking the 7 mg dose lost about 5 lbs (2.3kg), and those taking the 14 mg dose lost around 8.1 lbs (3.7 kg) over 26 weeks.
Mounjaro injection is the type 2 diabetes version of the weight-loss injection Zepbound. These drugs both contain the same active ingredient known as tirzepatide. Significant weight loss is also seen with Mounjaro, even though it's not approved for that use.
- In studies up to 52-weeks, when Mounjaro was used alone or with diabetes drugs, adults lost from 12 lb (5.5 kg) to 25 lb (11.4 kg) with the 5, 10 or 15 mg dose injected subcutaneously (under the skin) once weekly. In general, higher doses lead to greater weight loss.
Ozempic, Rybelsus or Mounjaro might be prescribed "off-label" by some doctors for weight loss in people who do not have type 2 diabetes, but your insurance may not cover it for this use. Off-label means your doctor may prescribe a drug for a use not specifically approved by the FDA or listed in the package insert.
It's best to check with your insurance company for payment for any weight loss treatment, as they are often not covered.
Related Questions
- Do you gain weight back after stopping Mounjaro?
- What is the Mounjaro Coupon or Savings Card?
- Mounjaro side effects you need to be aware of?
Are there any alternatives to Wegovy and Zepbound?
There are several other weight loss products approved by the FDA on the market, including another GLP-1 agent, appetite suppressants and lipase inhibitors.
Saxenda (liraglutide) is also a glucagon-like peptide-1 (GLP-1) receptor agonist injection and is approved for chronic weight management in adults and children 12 years of age and older.
In a 56-week study with over 3,700 participants, patients had an average weight loss of 4.5% of their weight compared to treatment with a placebo (inactive pill) at one year. In this trial, 62% of patients treated with Saxenda compared with 34% of patients treated with placebo lost at least 5% of their body weight.
- Adults: Approved to be used with diet and exercise for long-term weight management in adults with obesity (BMI ≥30 kg/m2) or overweight (BMI ≥27 kg/m2), in the presence of at least one weight-related medical condition (such as high blood pressure, type 2 diabetes, or elevated cholesterol levels).
- Children: Approved for use in adolescents 12 years and older with body weight above 60 kg (132 lb) and an initial BMI corresponding to 30 kg/m2 or greater for adults (obese) by international cut-offs, in addition to a reduced calorie diet and increased physical activity.
For eligible patients, this medicine is given as a subcutaneous (under the skin) injection once a day (in the abdomen, thigh, or upper arm). It comes as a prefilled injection pen and you (or a caregiver) can learn to self-inject this medicine so you can administer it at home.
If you have not lost 4% of your body weight after 16 weeks of treatment, your doctor may tell you to stop taking it. In children 12 years of age and older, your doctor may stop Saxenda after 12 weeks on the maintenance dose if BMI has not decreased by at least 1 percent.
Liraglutide is also approved for use in type 2 diabetes (under the brand name: Victoza) and to help reduce the risk of serious heart problems, but the two drugs should not be used together or with any other GLP-1 or GIP / GLP-1 receptor agonists like Wegovy, Ozempic or Zepbound.
A generic option for Victoza called liraglutide was also approved by the FDA in December 2024 but does not carry the indication for heart risk reduction.
Learn more: Saxenda side effects and warnings (in more detail)
How much weight can I lose with Qsymia?
In 2012, Qsymia (phentermine and extended-release topiramate) and Belviq (lorcaserin), two oral prescription medicines, were the first weight loss drugs approved in over a decade. However, Belviq was withdrawn from the market in 2020 due to safety concerns over a risk of cancer.
Qsymia is used for weight control in:
- Adults: Those who are obese (BMI of 30 kg/m2 or higher) OR those who are overweight (BMI of at least 27 kg/m2) and also have at least one weight-related condition, such as type 2 diabetes, high blood pressure, or high cholesterol.
- Children: Aged 12 years and older: with a body mass index (BMI) of 95th percentile or greater when standardized for age and sex.
Qsymia is an oral capsule taken once daily by mouth. In one year clinical trials, Qsymia weight loss in adults averaged 11.2 lbs to 24 lbs (5.1 kg to 10.9 kg). After 1 year of treatment with Qsymia, all dose levels resulted in statistically significant weight loss compared to placebo (a pill with no active medicine).
- In studies in adults with obesity, the percentage of patients losing at least 10% of their body weight were 19% (taking the 3.75 mg/23 mg dose) and 47% (taking the 15 mg/92 mg dose), compared to 7% of those taking a placebo.
- In studies in adults with obesity or overweight with risk factors like high blood pressure or high cholesterol, the percentage of patients losing at least 10% of their body weight were 37% (taking the 7.5 mg/46 mg dose) and 48% (taking 15 mg/92 mg dose), compared to 7% of those taking a placebo.
- In children 12 to 17 years of age with obesity, the percentage of patients with a reduction of greater than or equal to 10% of their BMI was 33.5% (taking the 7.5 mg/46 mg dose) and 44.4% (taking the 15 mg/92 mg dose) compared to 4.5% of those taking a placebo.
However, there was a high drop out rate of patients in the studies, ranging from 31% to 40% at one year. Patients may have dropped out due to side effects or lack of effect.
How does Qsymia work?
Qsymia is a combination of two drugs, phentermine and extended-released topiramate. Phentermine acts to suppress the appetite, and topiramate (normally used as an anti-seizure medicine), is used because it may help people feel full.
Qsymia is taken once daily in morning with or without food; patients should avoid an evening dose as it may keep them awake. Your doctor will slowly increase your dose over a 28-day period and will evaluate your weight loss after 12 to 24 weeks of treatment, depending upon your final dose.
If you have not lost at least 5% of your weight or BMI after 12 weeks, your doctor may decide to stop Qsymia treatment. Do not stop treatment on your own too quickly as it may cause seizures; always talk to your doctor first.
Qsymia is classified as CIV controlled substance due to the phentermine component, which is an amphetamine derivative. Drugs that are stimulants, like amphetamine can lead to misuse and dependence.
Learn more: Qsymia side effects and warnings (in more detail)
Why was Belviq withdrawn from the market?
The FDA requested the withdrawal of Belviq and Belviq XR (lorcaserin) from the US market in 2020 due to a reported increased risk of cancer.
Based on studies involving 12,000 people tracked for more than five years, the data showed that 7.1% of those taking a "dummy" placebo developed cancer, but that number rose to 7.7% among those taking Belviq. Cancer types included pancreatic, colorectal and lung cancer.
How does Contrave work for weight loss?
Contrave (bupropion and naltrexone) is an oral, extended-release form of two previously approved drugs, bupropion and naltrexone
- Bupropion is an antidepressant medicine that can also lower your appetite. Naltrexone, an opioid antagonist, is used to block the effects of narcotics or alcohol in addiction but may also curb hunger and food cravings
- When used together, these medications act on two areas of the brain to help with weight loss. It does not contain a stimulant and is not a controlled substance.
Contrave is approved to be used in adults with obesity (BMI: 30 kg/m2 or greater) or overweight (BMI: 27 kg/m2 or greater) and at least one weight-related condition such as high blood pressure, high cholesterol, or type 2 diabetes. Like other weight-loss drugs, it's used alongside diet and exercise.
- Studies in patients without diabetes showed that those using Saxenda lost about 4.1% of their weight compared to the group using placebo (an inactive pill) after one year. In this trial, 42% of patients treated with Contrave lost at least 5% of their body weight compared with 17% of patients treated with placebo.
- In another study with patients with type 2 diabetes, patients had an average weight loss of 2% over treatment with placebo at one year. In this trial, 36% of patients treated with Contrave lost at least 5% of their body weight compared to 18% of patients treated with placebo.
- Across studies when compared to placebo, weight loss with Saxenda ranged from 2 to 4.1 kg (4.4 to 9 lbs) over one year, on average.
After 12 weeks, if you have not lost at least 5% of your body weight, your doctor may decide to discontinue treatment.
Because it contain bupropion, Contrave can cause dose-related seizures and must not be used in patients who have seizure disorders.
Learn more: Contrave side effects and warnings (in more detail)
Is alli the same as Xenical?
alli (orlistat) is the over-the-counter (OTC) version of the FDA-approved prescription drug Xenical 120 mg but it comes in a lower 60 milligram (mg) strength. It's used in adults in conjunction with diet and regular exercise to promote weight loss.
alli decreases the absorption of dietary fat by about 25% and therefore reduces the number of calories absorbed.
Clinical trials have only shown it to be only modestly effective; in general, a weight loss of 3 to 5 lbs (1.4 to 2.3 kg) per year would be expected over and above what you might lose from dieting and exercise alone.
How is alli taken?
alli is taken as one 60 mg capsule three times a day with each meal containing fat. Do not take more than 3 capsules per day. Use alli in combination with diet and exercise. You should expect to lose most of your weight in the first 6 months.
It is also recommended to take a daily multivitamin at bedtime to help offset the loss of any fat-soluble vitamins. The vitamin should contain the fat soluble vitamins like A, D, E, K & beta carotene. Those with diabetes, thyroid disease or taking a blood thinner should consult with their doctor before using alli.
Side effects with alli may often hinder its use and include loose stools, oily spotting, gas, bowel incontinence, and rarely liver injury (jaundice). Contact your doctor right away if you have symptoms of liver problems, such as yellow skin or eyes, itching, brown urine or stomach pain.
Do not use this medicine if you are pregnant or breastfeeding.
Learn more: alli side effects and warnings (in more detail)
Stimulant weight loss drugs
Drugs that are considered stimulant weight loss drugs include:
- phentermine (Adipex-P)
- phendimetrazine (Bontril PDM)
- diethylpropion (generic only)
These stimulants are controlled substances approved for short-term use in weight loss, usually only up to 12 weeks maximum. As with other weight loss treatments, these drugs should be used in conjunction with ongoing diet and exercise to maintain weight loss.
Drugs that are stimulants (amphetamine-like) can lead to abuse and dependence with long-term use. Many doctors are reluctant to prescribe stimulants due to these risks and because of the availability of newer and safer weight loss options.
Often, the weight that is lost with stimulants will be regained when the medication is stopped. In contrast, Wegovy, Zepbound, alli, Contrave, Saxenda, and Qsymia are all approved for long-term (chronic) use to help maintain your weight loss over the long-term.
Are OTC herbal weight loss pills safe and effective?
It is tempting to buy OTC (over-the-counter) weight loss pills -- they seem quick, easy and may claim to be "natural". However, dietary or herbal supplements are not reviewed by the FDA like prescription medicines. They may contain unknown chemicals that can be dangerous or counterfeit. Be especially careful buying these products online and only buy from a reputable website.
The FDA will investigate OTC supplements if they appear to be causing harm. In fact, the FDA removed dietary products with the stimulant ephedra from the U.S. market in 2004 due to dangerous side effects such as heart attack, stroke and seizures.
Chromium, Green Tea extract, Hoodia, and Guar Gum are just a few of the herbal dietary supplements marketed for weight loss. Ask your pharmacist for recommendations when buying OTC products off of the shelf. Do not combined prescription weight loss medicines with OTC weight loss products, or herbal or dietary supplements without speaking to your healthcare provider first.
This is not all the information you need to know about weight loss treatment and does not take the place of your doctor’s directions. Your individual weight-loss results may vary. Discuss any medical questions you have with your doctor or other health care provider.
Learn more
- Atorvastatin (Lipitor): Top 12 Drug Facts You Need to Know
- Can You Mix Weight Loss Drugs and Alcohol?
- Cholesterol Medications and Alcohol: Can You Mix Them?
- Diabetes Medications and Alcohol Interactions
- Do blood pressure drugs interact with alcohol?
- OneTouch Blood Glucose Meters
- Side Effects of Weight Loss Drugs
- Statins for high cholesterol: Are the benefits worth the risk?
- Top 10 Diabetes Treatments You May Have Missed
- Weight Loss Drugs & Injections: What Are Your Options?
- Which Drugs Cause Weight Gain?
Treatment options
- Medications for Cardiovascular Risk Reduction
- Medications for Chronic Kidney Disease
- Medications for Diabetes Mellitus
- Medications for Gallbladder Disease
- Medications for High Blood Pressure
- Medications for High Cholesterol
- Medications for Hyperlipoproteinemia
Care guides
- Biliary Colic
- Biliary Dyskinesia
- Cholecystitis
- Chronic Hypertension
- Chronic Kidney Disease
- Diabetes and Pregnancy
- Diabetes and your Skin
Symptoms and treatments
- Diabetes mellitus
- High blood pressure (hypertension)
- High cholesterol (hypercholesterolemia)
- Secondary hypertension
Medicine.com guides (external)
Sources
- Malhotra A, Grunstein RR, Fietze I, et al; SURMOUNT-OSA Investigators. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N Engl J Med. 2024 Oct 3;391(13):1193-1205. doi: 10.1056/NEJMoa2404881. Epub 2024 Jun 21. Erratum in: N Engl J Med. 2024 Oct 17;391(15):1464. doi: 10.1056/NEJMx240005. PMID: 38912654; PMCID: PMC11598664
- Zepbound [prescribing information]. 12/2024. Indianapolis, IN. Eli Lilly and Co. Accessed Dec. 23, 2024 at https://pi.lilly.com/us/zepbound-uspi.pdf?s=pi
- Ozempic prescribing information. Revised 11/2024. Novo Nordisk. Plainsboro, NJ. Accessed Nov. 18, 2024 at https://www.novo-pi.com/ozempic.pdf
- Centers for Disease Control and Prevention (CDC). The High Obesity Program (HOP 2023): CDC-RFA-DP-23-0013 Accessed Oct 17, 2023.
- Zepbound [prescribing infomation]. 5/2024. Indianapolis, IN. Eli Lilly. Accessed Oct 18, 2024 at https://pi.lilly.com/us/zepbound-uspi.pdf?s=pi
- Wegovy [prescribing information]. 3/2024. Bagsvaerd, Denmark. Novo Nordisk. Accessed Oct 18, 2024 at https://www.novo-pi.com/wegovy.pdf
- Contrave [package insert]. 5/2024. Morristown, NJ. Nalpropion Pharmaceuticals. Accessed Oct 18, 2024 at https://www.curraxpharma.com/PI/Contrave-label-current.pdf/
- Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk Inc. Accessed Oct 18, 2024 at https://www.novo-pi.com/saxenda.pdf
- Qsymia [package insert]. Mountain View, CA: Vivus, Inc. Updated 6/2023. Accessed Oct 10, 2023 at https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/022580s023lbl.pdf
- FDA Requests Market Withdrawal of Diet Drug Belviq Due to Cancer Risk. US FDA. Accessed Oct 18, 2024 at https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
