Rhopressa: Package Insert / Prescribing Info
Package insert / product label
Generic name: netarsudil
Dosage form: ophthalmic solution
Drug class: Ophthalmic glaucoma agents
Medically reviewed by Drugs.com. Last updated on Sep 30, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
RHOPRESSA® (netarsudil ophthalmic solution) 0.02%, for topical ophthalmic use
Initial U.S. Approval: 2017
Recent Major Changes
Warnings and Precautions, Epithelial Corneal Edema (5.1) 9/2024
Indications and Usage for Rhopressa
RHOPRESSA® is a Rho kinase inhibitor indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. (1)
Rhopressa Dosage and Administration
One drop into the affected eye(s) once daily in the evening. (2)
Dosage Forms and Strengths
Ophthalmic solution containing netarsudil 0.02%. (3)
Contraindications
None. (4)
Adverse Reactions/Side Effects
The most common adverse reaction is conjunctival hyperemia (53%). Other common adverse reactions, approximately 20% include: corneal verticillata, instillation site pain, and conjunctival hemorrhage. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories, Inc. at 1‑800-757-9195, or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2024
Full Prescribing Information
1. Indications and Usage for Rhopressa
RHOPRESSA is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2. Rhopressa Dosage and Administration
The recommended dosage is one drop in the affected eye(s) once daily in the evening.
If one dose is missed, treatment should continue with the next dose in the evening. Twice a day dosing is not well tolerated and is not recommended. If RHOPRESSA is to be used concomitantly with other topical ophthalmic drug products to lower IOP, administer each drug product at least 5 minutes apart [see Patient Counseling Information (17)].
5. Warnings and Precautions
5.1 Epithelial Corneal Edema
Epithelial corneal edema, described as honeycomb or bullous, has been reported in some patients with pre-existing corneal stromal edema or following ocular procedures that could affect corneal endothelial function. Epithelial corneal edema typically resolves upon discontinuation of RHOPRESSA. Advise patients to notify their physician if they experience eye pain or decreased vision while using RHOPRESSA [see Adverse Reactions (6.2) and Patient Counselling Information (17)].
5.2 Bacterial Keratitis
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface [see Patient Counseling Information (17)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The most common ocular adverse reaction observed in controlled clinical studies with RHOPRESSA dosed once daily was conjunctival hyperemia which was reported in 53% of patients. Six percent of patients discontinued therapy due to conjunctival hyperemia. Other common (approximately 20%) ocular adverse reactions reported were: corneal verticillata, instillation site pain, and conjunctival hemorrhage. Instillation site erythema, corneal staining, blurred vision, increased lacrimation, erythema of eyelid, and reduced visual acuity were reported in 5-10% of patients.
Corneal Verticillata
Corneal verticillata occurred in approximately 20% of the patients in controlled clinical studies. The corneal verticillata seen in RHOPRESSA-treated patients were first noted at 4 weeks of daily dosing. This reaction did not result in any apparent visual functional changes in patients. Most corneal verticillata resolved upon discontinuation of treatment.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of RHOPRESSA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye disorders: Epithelial corneal edema has been reported in some patients with pre-existing corneal stromal edema or following ocular procedures (that could affect corneal endothelial function) [see Warnings and Precautions (5.1)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on RHOPRESSA use in pregnant women to inform any drug associated risk; however, systemic exposure to netarsudil from ocular administration is low [see Clinical Pharmacology (12.3)]. Intravenous administration of netarsudil to pregnant rats and rabbits during organogenesis did not produce adverse embryofetal effects at clinically relevant systemic exposures [see Data].
Data
Animal Data
Netarsudil administered daily by intravenous injection to rats during organogenesis caused abortions and embryofetal lethality at doses ≥0.3 mg/kg/day (126-fold the plasma exposure at the recommended human ophthalmic dose [RHOD], based on Cmax). The no-observed-adverse-effect-level (NOAEL) for embryofetal development toxicity was 0.1 mg/kg/day (40-fold the plasma exposure at the RHOD, based on Cmax).
Netarsudil administered daily by intravenous injection to rabbits during organogenesis caused embryofetal lethality and decreased fetal weight at 5 mg/kg/day (1480-fold the plasma exposure at the RHOD, based on Cmax). Malformations were observed at ≥3 mg/kg/day (1330-fold the plasma exposure at the RHOD, based on Cmax), including thoracogastroschisis, umbilical hernia and absent intermediate lung lobe. The NOAEL for embryofetal development toxicity was 0.5 mg/kg/day (214-fold the plasma exposure at the RHOD, based on Cmax).
8.2 Lactation
Risk Summary
There are no data on the presence of RHOPRESSA in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to netarsudil following topical ocular administration is low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of netarsudil would be present in maternal milk following topical ocular administration.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RHOPRESSA and any potential adverse effects on the breast-fed child from RHOPRESSA.
11. Rhopressa Description
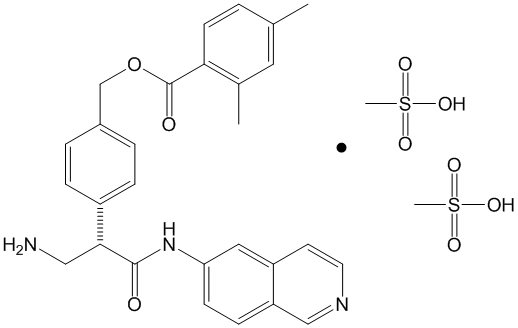
Netarsudil is a Rho kinase inhibitor. Its chemical name is (S)-4-(3-amino-1-(isoquinolin-6-yl-amino)-1-oxopropan-2-yl) benzyl 2,4-dimethylbenzoate dimesylate. The molecular formula of the free base is C28H27N3O3 and the molecular formula of the dimesylate is C30H35N3O9S2. The molecular weight of the free base is 453.54 and the molecular weight of the dimesylate is 645.74. The chemical structure is:
Netarsudil dimesylate is a light yellow-to-white powder that is freely soluble in water, soluble in methanol, sparingly soluble in dimethyl formamide, and practically insoluble in dichloromethane and heptane.
RHOPRESSA (netarsudil ophthalmic solution) 0.02% is supplied as a sterile, isotonic, buffered aqueous solution of netarsudil dimesylate with a pH of approximately 5 and an osmolality of approximately 295 mOsmol/kg. It is intended for topical application in the eye. Each mL of RHOPRESSA contains 0.2 mg of netarsudil (equivalent to 0.28 mg of netarsudil dimesylate). Benzalkonium chloride, 0.015%, is added as a preservative. The inactive ingredients are: boric acid, mannitol, sodium hydroxide to adjust pH, and water for injection.
12. Rhopressa - Clinical Pharmacology
12.1 Mechanism of Action
Netarsudil is a rho kinase inhibitor, which is believed to reduce IOP by increasing the outflow of aqueous humor through the trabecular meshwork. The exact mechanism is unknown.
12.3 Pharmacokinetics
Absorption
The systemic exposures of netarsudil and its active metabolite, AR-13503, were evaluated in 18 healthy subjects after topical ocular administration of RHOPRESSA 0.02% once daily (one drop bilaterally in the morning) for 8 days. There were no quantifiable plasma concentrations of netarsudil (lower limit of quantitation (LLOQ) 0.100 ng/mL) post dose on Day 1 and Day 8. Only one plasma concentration at 0.11 ng/mL for the active metabolite was observed for one subject on Day 8 at 8 hours post-dose.
Metabolism
After topical ocular dosing, netarsudil is metabolized by esterases in the eye.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of netarsudil. Netarsudil was not mutagenic in the Ames test, in the mouse lymphoma test, or in the in vivo rat micronucleus test. Studies to evaluate the effects of netarsudil on male or female fertility in animals have not been performed.
14. Clinical Studies
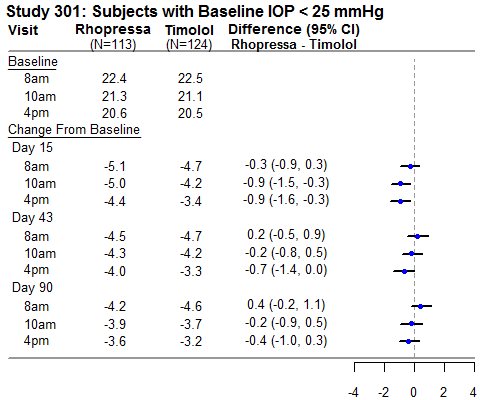
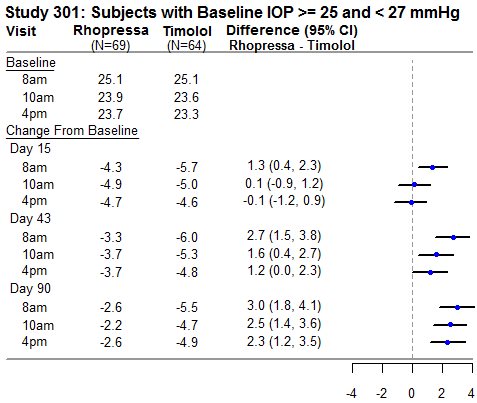
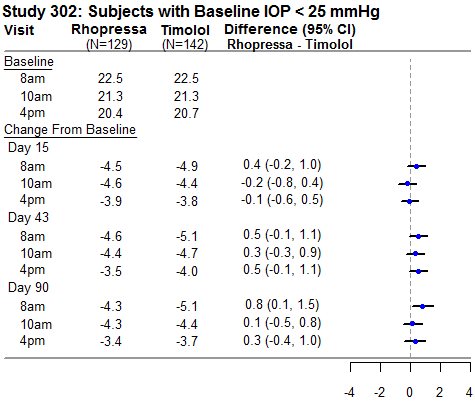
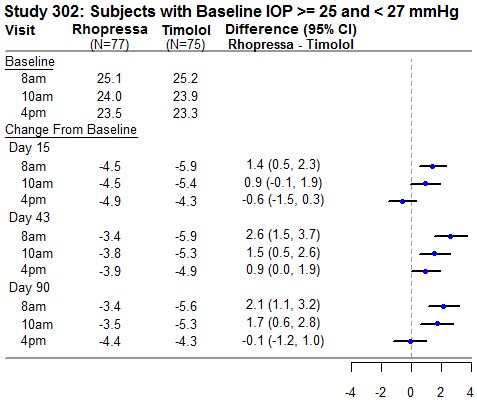
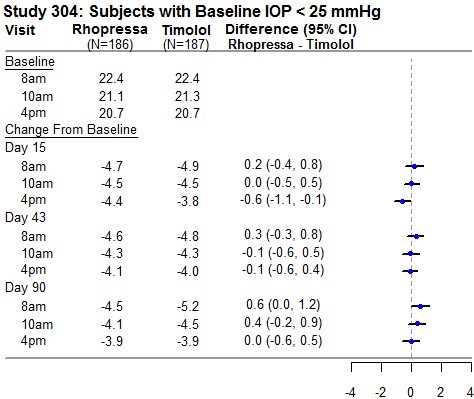
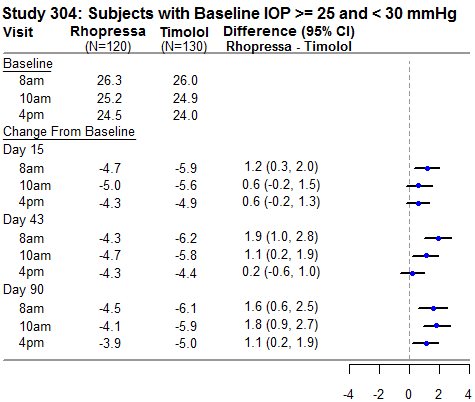
RHOPRESSA 0.02% was evaluated in three randomized and controlled clinical trials, namely AR-13324-CS301 (NCT 02207491, referred to as Study 301), AR-13324-CS302 (NCT 02207621, referred to as Study 302), and AR-13324-CS304 (NCT 02558374, referred to as Study 304), in patients with open-angle glaucoma or ocular hypertension. Studies 301 and 302 enrolled subjects with baseline IOP lower than 27 mmHg and Study 304 enrolled subjects with baseline IOP lower than 30 mmHg. The treatment duration was 3 months in Study 301, 12 months in Study 302, and 6 months in Study 304.
The three studies demonstrated up to 5 mmHg reductions in IOP for subjects treated with RHOPRESSA 0.02% once daily in the evening. For patients with baseline IOP < 25 mmHg, the IOP reductions with RHOPRESSA 0.02% dosed once daily were similar to those with timolol 0.5% dosed twice daily (see Table 1). For patients with baseline IOP equal to or above 25 mmHg, however, RHOPRESSA 0.02% resulted in smaller mean IOP reductions at the morning time points than timolol 0.5% for study visits on Days 43 and 90; the difference in mean IOP reduction between the two treatment groups was as high as 3 mmHg, favoring timolol.
Table 1: Mean IOP Change from Baseline of Study Eye (mmHg) by Visit and Time
This table was produced based on the observed data from all randomized subjects who did not have major protocol violations. The treatment differences and two-sided CIs for comparing Rhopressa QD vs Timolol BID 0.5% were based on Analysis of Covariance (ANCOVA) adjusted for baseline IOP.
16. How is Rhopressa supplied
RHOPRESSA® (netarsudil ophthalmic solution) 0.02% (0.2 mg per mL) is supplied sterile in opaque white low density polyethylene bottles and tips with white polypropylene caps.
2.5 mL fill in a 4 mL container
NDC # 70727-497-25
Storage: Store at 2°C to 8°C (36°F to 46°F) until opened. After opening, the product may be kept at 2°C to 25°C (36°F to 77°F) for up to 6 weeks. If after opening the product is kept refrigerated at 2°C to 8°C (36°F to 46°F), then the product can be used until the expiration date stamped on the bottle. During shipment, the bottle may be maintained at temperatures up to 40°C (104°F) for a period not exceeding 14 days.
17. Patient Counseling Information
Handling the Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions [see Warnings and Precautions (5.2)].
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma, infection, or decreased vision with or without eye pain), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of RHOPRESSA.
Use with Contact Lenses
Advise patients that RHOPRESSA contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of RHOPRESSA and may be reinserted 15 minutes following its administration.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least 5 minutes between applications.
Missed Dose
Advise patients that if one dose is missed, treatment should continue with the next dose in the evening.
@ 2024 Alcon Inc.
U.S. Pat.: www.alconpatents.com
Manufactured for: ALCON LABORATORIES, INC.
6201 South Freeway Fort Worth,
Texas 76134 USA
1-800-757-9195
Alcon.medinfo@alcon.com
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 70727-497-25
rhopressa®
(netarsudil ophthalmic
solution) 0.02%
For topical application
in the eye
Sterile
Rx only
2.5 mL
Alcon
Usual dosage:
One drop in the affected
eye(s) once daily in the
evening.
Each mL Rhopressa®
ophthalmic solution contains:
Active: netarsudil mesylate
0.285 mg
Preservative:
benzalkonium chloride
0.15 mg
Inactives: mannitol, boric
acid, sodium hydroxide to
adjust pH and water for
injection.
Manufactured for:
ALCON LABORATORIES, INC.
Fort Worth, TX 76134 USA
U.S. Pat: www.alconpatents.com
Store at 2°C - 8°C
(36°F - 46°F) until opened.
After opening, the product
may be kept at 2°C - 25°C
(36°F - 77°F) for up to 6
weeks. If after opening the
product is kept refrigerated at
2°C - 8°C (36°F - 46°F), then
the product can be used until
the expiration date stamped
on the bottle. During
shipment, the bottle may be
maintained at temperatures
up to 40°C (104°F) for a
period not exceeding 14 days.
41079
NDC 70727-497-25 For topical application in the eye.
rhopressa®
(netarsudil ophthalmic
solution) 0.02%
Rx only
Sterile
2.5 mL Alcon 41077
See carton for storage conditions.
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, TX 76134
LOT:
EXP:

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 70727-497-99
SAMPLE - Not for Resale
rhopressa®
(netarsudil ophthalmic
solution) 0.02%
For topical application in the eye
Sterile Rx only
2.5 mL
Alcon
Usual dosage:
One drop in the affected
eye(s) once daily in the
evening.
Each mL Rhopressa®
ophthalmic solution contains:
Active: netarsudil mesylate
0.285 mg
Preservative:
benzalkonium chloride
0.15 mg
Inactives: mannitol, boric
acid, sodium hydroxide to
adjust pH and water for
injection.
Manufactured for:
ALCON LABORATORIES, INC.
Fort Worth, TX 76134 USA
U.S. Pat: www.alconpatents.com
Store at 2°C - 8°C
(36°F - 46°F) until opened.
After opening, the product
may be kept at 2°C - 25°C
(36°F - 77°F) for up to 6
weeks. If after opening the
product is kept refrigerated at
2°C - 8°C (36°F - 46°F), then
the product can be used until
the expiration date stamped
on the bottle. During
shipment, the bottle may be
maintained at temperatures
up to 40°C (104°F) for a
period not exceeding 14 days.
41080
NDC 70727-497-25 SAMPLE - Not for Resale For topical application in the eye.
rhopressa®
(netarsudil ophthalmic
solution) 0.02%
Rx only
Sterile
2.5 mL Alcon 41078
See carton for
storage conditions.
Manufactured for:
Alcon Laboratories, Inc.
Fort Worth, TX 76134
LOT:
EXP:

| RHOPRESSA
netarsudil solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
Frequently asked questions
More about Rhopressa (netarsudil ophthalmic)
- Compare alternatives
- Pricing & coupons
- Reviews (36)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: ophthalmic glaucoma agents
- Breastfeeding
- En español