Jynneos: Package Insert / Prescribing Info
Package insert / product label
Generic name: vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen
Dosage form: injection, suspension
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Apr 22, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
JYNNEOS (Smallpox and Mpox Vaccine, Live, Non-replicating) injectable suspension for subcutaneous use
Initial U.S. Approval: 2019
Recent Major Changes
Dosage and Administration (2) 03/2025
Indications and Usage for Jynneos
JYNNEOS is a vaccine indicated for prevention of smallpox and mpox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection. (1)
Jynneos Dosage and Administration
For subcutaneous use. (2)
JYNNEOS is supplied in two presentations:
One-Vial Presentation:
The one-vial presentation contains JYNNEOS in a single vial with a yellow cap and does not require reconstitution before use. (2.1, 2.2)
Two-Vial Presentation:
The two-vial presentation includes a vial with a yellow cap containing Lyophilized Antigen Component and a vial with a blue cap containing Diluent Component. The Lyophilized Antigen Component must be reconstituted with the Diluent Component to form JYNNEOS prior to administration. (2.1, 2.2)
Administer two doses (0.5 mL each) 4 weeks apart. (2.4)
Dosage Forms and Strengths
Injectable suspension. A single dose is 0.5 mL. (3)
Adverse Reactions/Side Effects
- •
- In smallpox vaccine-naïve healthy adults, the most common (> 10%) solicited injection site reactions were pain (84.9%), redness (60.8%), swelling (51.6%), induration (45.4%), and itching (43.1%); the most common solicited systemic adverse reactions were muscle pain (42.8%), headache (34.8%), fatigue (30.4%), nausea (17.3%) and chills (10.4%). (6.1)
- •
- In healthy adults previously vaccinated with a smallpox vaccine, the most common (> 10%) solicited injection site reactions were redness (80.9%), pain (79.5%), induration (70.4%), swelling (67.2%), and itching (32.0%); the most common solicited systemic adverse reactions were fatigue (33.5%), headache (27.6%), and muscle pain (21.5%). (6.1)
- •
- The frequencies of solicited local and systemic adverse reactions among adults with HIV-infection and adults with atopic dermatitis were generally similar to those observed in healthy adults. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic at toll-free phone 1-833-365-9596 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2025
Full Prescribing Information
1. Indications and Usage for Jynneos
JYNNEOS is a vaccine indicated for prevention of smallpox and mpox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection.
2. Jynneos Dosage and Administration
For subcutaneous use.
2.1 JYNNEOS Presentations
JYNNEOS is supplied in two presentations, a one vial-presentation and a two-vial presentation.
One-Vial Presentation
The one-vial presentation contains JYNNEOS in a single vial with a yellow cap and does not require reconstitution before use.
Two-Vial Presentation
The two-vial presentation includes a vial with a yellow cap containing Lyophilized Antigen Component (Vial A) and a vial with a blue cap containing Diluent Component (Vial B). The Lyophilized Antigen Component must be reconstituted with the Diluent Component to form JYNNEOS prior to administration.
2.2 Preparation
Instructions for JYNNEOS One-Vial Presentation
Allow the vaccine to thaw and reach room temperature before use. Once thawed, the vaccine may be kept at +2°C to +8°C (+36°F to +46°F) for 4 weeks. Protect from light during storage.
Do not refreeze.
Swirl the vial gently before use for at least 30 seconds. Withdraw a dose of 0.5 mL into a sterile syringe for injection.
Reconstitution Instructions for JYNNEOS Two-Vial Presentation
Reconstitute the Lyophilized Antigen Component (Vial A, yellow cap) with Diluent Component (Vial B, blue cap) to form JYNNEOS, as described in Figure 1 through Figure 4.
After reconstitution, the vaccine may be stored at +2°C to +8°C (+36°F to +46°F) for 12 hours. Protect from light during storage.
2.3 Administration
JYNNEOS is a milky, light yellow to pale white colored suspension. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the vaccine should not be administered.
Administer each 0.5 mL dose of JYNNEOS subcutaneously.
5. Warnings and Precautions
5.1 Severe Allergic Reactions
Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of JYNNEOS.
Persons who experienced a severe allergic reaction following a previous dose of JYNNEOS or following exposure to any component of JYNNEOS may be at increased risk for severe allergic reactions after JYNNEOS. The risk for a severe allergic reaction should be weighed against the risk for disease due to smallpox or mpox.
5.2 Syncope
Syncope (fainting) has been reported following vaccination with JYNNEOS. Procedures should be in place to avoid injury from fainting.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine, and may not reflect the rates observed in practice. There is the possibility that broad use of JYNNEOS could reveal adverse reactions not observed in clinical trials.
Safety data accrued with the one-vial presentation is relevant to the two-vial presentation because each contains the same live, attenuated, non-replicating orthopoxvirus and is manufactured using a similar process.
The overall clinical trial program included 23 studies (14 studies with the one-vial presentation, 7 studies with the two-vial presentation, and 2 studies with both one-vial and two-vial presentations) and a total of 8,988 participants 18 through 80 years of age who received at least 1 dose of JYNNEOS (8,222 smallpox vaccine-naïve and 766 smallpox vaccine-experienced participants). Of the 8,988 participants, 6,459 participants received the one-vial presentation and 2,529 participants received the two-vial presentation.
Solicited Adverse Reactions
Solicited Adverse Reactions in Smallpox Vaccine-Naïve Individuals:
The safety of JYNNEOS one-vial presentation in smallpox vaccine-naïve participants was evaluated in Study 1 [1], a randomized, double-blind, placebo-controlled study conducted in the US in which vaccinia-naïve adults ages 18 to 40 years received either two doses of JYNNEOS (N=3,003), or two injections of Tris-Buffered Saline (placebo, N=1,002) four weeks apart.
In the total study population, the mean age was 28 years; 47.9% of the participants were men; 77.4% were white/Caucasian, 17.8% black/African American, 1.9% Asian, 0.5% American Indian/Alaska Native, 0.4% Native Hawaiian/Other Pacific, 1.9% other racial groups; and 11.4% of participants were of Hispanic/Latino ethnicity. The demographic distribution was similar in the JYNNEOS and placebo groups.
In Study 1, participants were monitored for local and systemic adverse reactions using diary cards for an 8-day period starting on the day of each vaccination. The frequencies of solicited local and systemic adverse reactions following any dose of JYNNEOS are presented in Table 1.
| Reaction | JYNNEOS
N=2943 % | Placebo
N=980 % |
|---|---|---|
| x NCT01144637 a Grade 3 pain defined as spontaneously painful b Grade 3 itching, muscle pain, headache, fatigue, nausea and chills defined as preventing routine daily activities c Fever defined as oral temperature ≥ 100.4°F (≥ 38°C), Grade ≥ 3 fever defined as ≥ 102.2°F (≥ 39.0°C) N: Number of participants in the specified treatment group |
||
|
Local (Injection site) |
-- |
-- |
|
Pain |
84.9 |
19.1 |
|
Pain, Grade 3a |
7.4 |
1.0 |
|
Redness |
60.8 |
17.7 |
|
Redness ≥ 100 mm |
1.5 |
0.0 |
|
Swelling |
51.6 |
5.6 |
|
Swelling ≥ 100 mm |
0.8 |
0.0 |
|
Induration |
45.4 |
4.6 |
|
Induration ≥ 100 mm |
0.3 |
0.0 |
|
Itching |
43.1 |
11.7 |
|
Itching, Grade 3b |
1.6 |
0.2 |
|
Systemic |
-- |
-- |
|
Muscle Pain |
42.8 |
17.6 |
|
Muscle Pain, Grade 3b |
2.6 |
0.7 |
|
Headache |
34.8 |
25.6 |
|
Headache, Grade 3b |
2.4 |
2.1 |
|
Fatigue |
30.4 |
20.5 |
|
Fatigue, Grade 3b |
3.0 |
1.3 |
|
Nausea |
17.3 |
13.1 |
|
Nausea, Grade 3b |
1.5 |
1.2 |
|
Chills |
10.4 |
5.8 |
|
Chills, Grade 3b |
1.0 |
0.3 |
|
Feverc |
1.7 |
0.9 |
|
Fever, Grade ≥ 3c |
0.2 |
0.0 |
In Study 1, the majority of solicited local and systemic adverse reactions reported with JYNNEOS had a median duration of 1 to 6 days. In general, there were similar proportions of participants reporting solicited local or systemic reactions of any severity after Dose 2 of JYNNEOS compared with Dose 1, with the exception of injection site pain, which was more commonly reported following Dose 1 (79.3%) than Dose 2 (69.9%).
The two presentations of JYNNEOS (one-vial and two-vial) were compared in Study 2 [2], a randomized, double-blind, multicenter study conducted in the US in which vaccinia-naïve adults ages 18 to 55 years received two doses of either two-vial presentation (N=325) or one-vial presentation (N=327) JYNNEOS four weeks apart.
The mean age was approximately 28 years for both study groups; 46.5% of the participants were men; 74.7% were White/Caucasian, 22.3% Black/African American, 0.8% Oriental/Asian, 0.2% American Indian/Alaska Native, 0.3% Native Hawaiian/ Pacific Islander, 1.5% multiple racial groups, and 0.3% other racial groups; and 29.2% of participants were of Hispanic/Latino ethnicity. The demographic distribution was similar in the two JYNNEOS treatment groups.
In Study 2, participants were monitored for local and systemic adverse reactions for an 8-day period starting on the day of each vaccination. The frequencies of solicited local and systemic adverse reactions following any dose of JYNNEOS are presented in Table 2.
| x NCT01668537 a Grade 3 pain defined as spontaneously painful/prevention of normal activity. b Grade 3 itching defined as preventing routine daily activities. c Grade 3 muscle pain, headache, fatigue, nausea and chills defined as severe; preventing routine daily activities d Fever defined as oral temperature ≥ 100.4°F (≥ 38°C), Grade ≥ 3 fever defined as ≥ 102.2°F (≥ 39.0°C) N: Number of participants in the specified treatment group |
||
|
Reaction |
JYNNEOS One-vial Presentation N=322 % |
JYNNEOS Two-vial Presentation N=321 % |
|
Any Local (Injection site) |
93.2 |
94.1 |
|
Local (Injection site) ≥ Grade 3 |
13.0 |
24.0 |
|
Pain |
85.1 |
90.3 |
|
Pain, Grade 3a |
6.8 |
10.9 |
|
Redness |
76.7 |
79.4 |
|
Redness ≥ 100 mm |
5.0 |
14.6 |
|
Swelling |
65.2 |
69.5 |
|
Swelling≥ 100 mm |
3.1 |
7.2 |
|
Induration |
65.5 |
66.4 |
|
Induration≥ 100 mm |
1.6 |
1.9 |
|
Itching |
55.0 |
58.9 |
|
Itching, Grade 3b |
2.2 |
3.4 |
|
Any Systemic |
56.5 |
57.6 |
|
Systemic ≥ Grade 3 |
5.9 |
5.9 |
|
Headache |
38.8 |
34.9 |
|
Headache, Grade 3c |
2.5 |
2.2 |
|
Fatigue |
35.1 |
31.8 |
|
Fatigue, Grade 3c |
4.7 |
2.8 |
|
Muscle Pain |
21.1 |
18.4 |
|
Muscle Pain, Grade 3c |
0.3 |
1.6 |
|
Nausea |
17.7 |
15.9 |
|
Nausea, Grade 3c |
1.2 |
0.6 |
|
Chills |
11.8 |
10.9 |
|
Chills, Grade 3c |
0.6 |
1.6 |
|
Feverd |
7.1 |
10.3 |
|
Fever, Grade ≥ 3d |
0.9 |
0.6 |
In Study 2, solicited systemic adverse reactions reported with both presentations had a similar median duration of 1 to 3 days. Solicited local adverse reactions reported with both presentations had a similar median duration of 4 to 6 days, except for injection site induration. The median duration of injection site induration was 11 days for the one-vial presentation and 14 days for the two-vial presentation.
Solicited Adverse Reactions in Persons Previously Vaccinated with a Smallpox Vaccine:
Three studies (Study 3, Study 4, and Study 5 [3-5]) conducted in the US and Germany evaluated the safety of JYNNEOS one-vial presentation in 409 persons previously vaccinated with a smallpox vaccine who received one or two doses of JYNNEOS (mean age 39 years, range 20-80 years; 59% women; 98.8% white/Caucasian; 0.7% Asian; 0.5% black/African American). Participants were monitored for local and systemic adverse reactions using diary cards for an 8-day period starting on the day of each vaccination. Across all three studies, solicited local adverse reactions reported following any dose of JYNNEOS were redness (80.9%), pain (79.5%), induration (70.4%), swelling (67.2%), and itching (32.0%) at the injection site; solicited systemic adverse reactions reported following any dose of JYNNEOS were fatigue (33.5%), headache (27.6%), muscle pain (21.5%), nausea (9.8%), chills (0.7%), and fever (0.5%).
Solicited Adverse Reactions in HIV-infected Individuals:
The safety of JYNNEOS one-vial presentation in HIV-infected participants was evaluated in Study 6 [6], an open label trial conducted in the US that included 351 HIV-infected smallpox vaccine-naïve participants, 131 HIV-infected participants who previously received smallpox vaccine, 88 non-HIV-infected smallpox vaccine-naïve participants and 9 non-HIV-infected participants who had previously received a smallpox vaccine. The demographic distribution (race, ethnicity, and sex) of HIV-infected smallpox vaccine-naïve participants and those who had previously received smallpox vaccine were similar and overall were 17.0% women; 45.8% white/Caucasian; 0.4% Asian; 33.2% black/African American; 19.0% Hispanic/Latino ethnicity; the HIV-infected smallpox vaccine-naïve group tended to be younger (mean age 37 years) compared to those who had previously received a smallpox vaccine (mean age 45 years). Participants had CD4 counts ≥ 200 and ≤ 750 cells/μL at study entry.
Solicited local and systemic adverse reactions were reported at similar or lower frequencies in HIV-infected smallpox vaccine-naïve participants as compared to those seen in non-HIV-infected smallpox vaccine-naive participants in this study.
In HIV-infected participants with previous smallpox vaccine exposure, fever and chills were reported in 1.5% and 8.4% of participants respectively. Frequencies of other solicited local and systemic adverse reactions in this population were similar to those reported in Studies 3-5 in non-HIV-infected participants who had previously received smallpox vaccination.
The safety of JYNNEOS two-vial presentation in HIV-infected participants was evaluated in Study 7 [7], an open label trial conducted in the US that included 30 HIV-infected smallpox vaccine-naïve participants, 61 HIV-infected participants who previously received smallpox vaccine, 30 non-HIV-infected smallpox vaccine-naïve participants, and 30 non-HIV-infected participants who had previously received a smallpox vaccine. The demographic distribution (race, ethnicity, and sex) of HIV-infected smallpox vaccine-naïve participants and those who had previously received smallpox vaccine were similar. Overall for HIV-infected participants, 29.7% of participants were women; 58.2% were white/Caucasian; 40.7% black/African American; 1.1% other race; Hispanic/Latino ethnicity was not reported; the HIV-infected smallpox vaccine-naïve group tended to be younger (mean age approximately 31 years) compared to those who had previously received a smallpox vaccine (mean age approximately 43 years). Participants had CD4 counts > 350 cells/μL at study entry.
Solicited local and systemic adverse reactions were reported at similar or lower frequencies in HIV-infected smallpox vaccine-naïve participants as compared to those seen in non-HIV-infected smallpox vaccine-naive participants who received the two-vial presentation in this study with the exception of fatigue (40.7% HIV-infected, 36.7% non-HIV infected).
Study 7 also evaluated the safety of a single dose of JYNNEOS two-vial presentation in 61 HIV-infected participants who previously received a smallpox vaccine and 30 non-HIV-infected participants who previously received a smallpox vaccine. Overall for participants who previously received a smallpox vaccine, the mean age was approximately 44 years, range 35-55 years; 44% women; 63.7% white/Caucasian; 34.1% black/African American; 2.2% other race. Participants were monitored for local and systemic adverse reactions for an 8-day period starting on the day of each vaccination. Solicited local adverse reactions reported among both HIV-infected and non HIV-infected participants previously vaccinated with a smallpox vaccine following a single dose of JYNNEOS two-vial presentation were redness (54.9%), pain (84.6%), induration (38.5%), swelling (45.1%), and itching (18.7%) at the injection site; solicited systemic adverse reactions reported following any dose of JYNNEOS were fatigue (38.5%), headache (37.4%), muscle pain (42.9%), nausea (27.5%), chills (16.5%), and fever (2.2%).
In HIV-infected participants with previous smallpox vaccine exposure who received the two-vial presentation, solicited local and systemic adverse reactions were reported at similar or lower frequencies compared to those reported in non-HIV-infected participants who previously received smallpox vaccination in this study.
Solicited Adverse Reactions in Individuals with Atopic Dermatitis:
The safety of JYNNEOS one-vial presentation in smallpox vaccine-naïve participants with currently active or a history of atopic dermatitis (AD) was evaluated in a multicenter, open-label clinical study (Study 8 [8]) conducted in the US and Mexico that included 350 participants with AD and 282 participants without AD. In the overall study the mean age of participants was 27 years (range 18-42 years), and participants were 59.0% women, 39.4% white/Caucasian, 10.9% Asian, 9.0% black/African American, 2.2% Other, and 38.4% Hispanic/Latino ethnicity. Demographic distribution was similar between participants with and without AD. In participants with AD, solicited local and systemic adverse reactions were reported at similar frequencies as those in participants without AD in this study, with the exception of redness (61.2% with AD vs. 49.3% without AD), swelling (52.2% with AD vs. 40.8% without AD), chills (15.9% with AD vs. 7.8% without AD) and headache (47.2% with AD vs. 34.8% without AD).
The safety of JYNNEOS two-vial presentation in smallpox vaccine-naïve participants with currently active or a history of atopic dermatitis (AD) was evaluated in an open-label, controlled clinical study (Study 9 [9]) conducted in Germany that included 31 participants with AD and 29 participants without AD. In the overall study, the mean age was approximately 25 years (range 19-34 years), and participants were 48.3% women, 95% white/Caucasian, 3.3% Asian, 1.7% black/African American; Hispanic/Latino ethnicity was not reported. The demographic distribution was similar between participants with AD and participants without AD. In participants with AD, solicited local and systemic adverse reactions were reported at similar frequencies as those in participants without AD in this study.
The following solicited local and systemic adverse reactions were reported at a greater frequency in participants with AD than those without AD in this study: redness (96.8% with AD vs. 89.7% without AD), itching (25.8% with AD vs. 13.8% without AD), fatigue (67.7% with AD vs. 51.7% without AD), headache (54.8% with AD vs. 44.8% without AD), myalgia (29% with AD vs. 24.1% without AD), and nausea (16.1% with AD vs. 10.3% without AD).
Serious Adverse Events
The integrated analyses of serious adverse events (SAEs) pooled safety data across 23 studies, which included a total of 8,222 smallpox vaccine-naïve participants and 766 smallpox vaccine-experienced participants who received at least 1 dose of JYNNEOS and 1,219 smallpox vaccine-naïve participants and 1 smallpox vaccine-experienced participant who received placebo only. SAEs were monitored from the day of the first study vaccination through at least 6 months after the last study vaccination.
Overall, SAEs were reported to occur in 1.5% (136/8,988) of JYNNEOS (one-vial or two-vial presentation) recipients and 1.4% (17/1,220) of placebo recipients. Among the smallpox vaccine-naïve participants, SAEs were reported for 1.3% (78/5,805) of JYNNEOS one-vial presentation recipients, 1.7% (40/2,417) of JYNNEOS two-vial presentation participants, and 1.4% (17/1,219) of placebo recipients. Among the smallpox vaccine-experienced participants enrolled in studies, SAEs were reported for 2.3% (15/654) of JYNNEOS one-vial presentation recipients and 2.7% (3/112) of JYNNEOS two-vial presentation recipients. Across all studies, a causal relationship to JYNNEOS could not be excluded for 5 SAEs, all non-fatal, which included Crohn’s disease, sarcoidosis, extraocular muscle paresis, throat tightness, and hemolytic anemia.
Cardiac Adverse Events of Special Interest
Evaluation of cardiac adverse events of special interest (AESIs) included any cardiac signs or symptoms, ECG changes determined to be clinically significant, or troponin-I elevated above 2 times the upper limit of normal. In the 23 studies, participants were monitored for cardiac-related signs or symptoms through at least 6 months after the last vaccination.
The numbers of JYNNEOS and placebo recipients, respectively, with troponin-I data were: baseline level (7,505 and 1,203); level two weeks after first dose (6,284 and 1,166); level two weeks after second dose (1,684 and 193); unscheduled visit, including for clinical evaluation of suspected cardiac adverse events (501 and 60).
Overall, cardiac AESIs were reported to occur in 1.3% (112/8,988) of JYNNEOS (one-vial or two-vial presentation) recipients and 0.2% (3/1,220) of placebo recipients. The higher proportion of JYNNEOS one-vial presentation recipients who experienced cardiac AESIs was driven by 28 cases of asymptomatic post-vaccination elevation of troponin-I in two studies: Study 6, which enrolled 482 HIV-infected participants and 97 healthy participants, and Study 8, which enrolled 350 participants with atopic dermatitis and 282 healthy participants. An additional 127 cases of asymptomatic post-vaccination elevation of troponin-I above the upper limit of normal but not above 2 times the upper limit of normal were documented in JYNNEOS recipients throughout the clinical development program, 124 of which occurred in Study 6 and Study 8. Proportions of participants with troponin-I elevations were similar between healthy and HIV-infected participants in Study 6 and between healthy and atopic dermatitis participants in Study 8. A different troponin assay was used in these two studies compared to the other studies, and these two studies had no placebo controls. The clinical significance of these asymptomatic post-vaccination elevations of troponin-I is unknown.
Among the cardiac AESIs reported, 6 cases (<0.1%) were considered to be causally related to JYNNEOS vaccination and included tachycardia, electrocardiogram T wave inversion, electrocardiogram abnormal, electrocardiogram ST segment elevation, electrocardiogram T wave abnormal, and palpitations.
None of the cardiac AESIs considered causally related to study vaccination were considered serious.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of JYNNEOS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Immune System Disorders: hypersensitivity reactions, including angioedema, rash, and urticaria
Nervous System Disorders: dizziness, syncope, facial paralysis (Bell’s palsy), acute disseminated encephalomyelitis, myelin oligodendrocyte glycoprotein antibody-associated disease, bilateral optic neuritis
General disorders and administration site conditions: injection site warmth, injection site vesicles
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Available human data on JYNNEOS administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
The effect of JYNNEOS on embryo-fetal and post-natal development was evaluated in four developmental toxicity studies conducted in female rats and rabbits. In two studies, rats were administered a single human dose of JYNNEOS (0.5 mL) once prior to mating and on one or two occasions during gestation. In the third study, rats were administered a single human dose of JYNNEOS (0.5 mL) on two occasions during gestation. In the fourth study, rabbits were administered a single human dose of JYNNEOS (0.5 mL) once prior to mating and on two occasions during gestation. These animal studies revealed no evidence of harm to the fetus [see Data].
Data
Animal Data
Developmental toxicity studies were conducted in female rats and rabbits. In one study, female rabbits were administered a single human dose of JYNNEOS (0.5 mL) by the subcutaneous route on three occasions: prior to mating, and on gestation days 0 and 14. Three studies were conducted in female rats administered a single human dose of JYNNEOS (0.5 mL) by the subcutaneous route on two or three occasions: prior to mating, and on gestation days 0 and 14; or prior to mating, and on gestation day 0; or on gestation days 0 and 6. No vaccine-related fetal malformations or variations and adverse effects on female fertility or pre-weaning development were reported in these studies.
8.2 Lactation
Risk Summary
It is not known whether JYNNEOS is excreted in human milk. Data are not available to assess the effects of JYNNEOS in the breastfed infant or on milk production/excretion.
The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for JYNNEOS and any potential adverse effects on the breastfed child from JYNNEOS or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness of JYNNEOS have not been established in individuals less than 18 years of age.
8.5 Geriatric Use
Forty-two smallpox vaccine-experienced adults 65 to 80 years of age received at least one dose of JYNNEOS (Study 5).
Clinical studies of JYNNEOS did not include sufficient numbers of individuals aged 65 and over to determine whether they respond differently from younger individuals.
11. Jynneos Description
JYNNEOS (Smallpox and Mpox Vaccine, Live, Non-replicating) is a sterile injectable suspension administered subcutaneously.
JYNNEOS is a live vaccine produced from the strain Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN), an attenuated, non-replicating orthopoxvirus. MVA-BN is grown in primary Chicken Embryo Fibroblast (CEF) cells suspended in a serum-free medium containing no material of direct animal origin, harvested from the CEF cells, purified and concentrated by several Tangential Flow Filtration (TFF) steps including benzonase digestion.
Each 0.5 mL dose of JYNNEOS is formulated to contain 0.5 x 108 to 3.95 x 108 infectious units of MVA-BN live virus. Each 0.5 mL dose contains 0.61 mg of Tris (tromethamine), 4.09 mg sodium chloride, and may contain residual amounts of host-cell DNA (≤20 mcg), protein (≤500 mcg), benzonase (≤0.0025 mcg), gentamicin (≤0.400 mcg), and ciprofloxacin (≤0.005 mcg). In addition, JYNNEOS vaccine reconstituted with Diluent Component (Water for Injection) contains 9.45 mg dextran 40, 22.5 mg sucrose, and 0.054 mg potassium L-glutamate monohydrate as stabilizers. After preparation [see Dosage and Administration (2.2)], JYNNEOS is a milky, light yellow to pale white colored suspension.
JYNNEOS is formulated without preservatives. The vial stoppers are not made with natural rubber latex.
12. Jynneos - Clinical Pharmacology
12.1 Mechanism of Action
JYNNEOS is an attenuated, live, non-replicating smallpox and mpox vaccine that elicits humoral and cellular immune responses to orthopoxviruses. Vaccinia neutralizing antibody responses in humans were evaluated to establish the effectiveness of JYNNEOS for prevention of smallpox and mpox.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
JYNNEOS has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male fertility in animals. Developmental toxicity studies conducted in rats and rabbits vaccinated with JYNNEOS revealed no evidence of impaired female fertility [see Use in Specific Populations (8.1)].
13.2 Animal Toxicology and/or Pharmacology
The efficacy of JYNNEOS to protect cynomolgus macaques (Macaca fascicularis) against a monkeypox virus (MPXV) challenge was evaluated in several studies. Animals were administered Tris-Buffered Saline (placebo) or JYNNEOS (1 x 108 TCID50) subcutaneously on day 0 and day 28. On day 63, animals were challenged with MPXV delivered by aerosol (3 x 105 pfu), intravenous (5 x 107 pfu) or intratracheal (5 x 106 pfu) route. Across all studies, 80-100% of JYNNEOS-vaccinated animals survived compared to 0-40% of control animals.
14. Clinical Studies
14.1 Vaccine Effectiveness
Vaccine effectiveness against smallpox was inferred by comparing the immunogenicity of JYNNEOS to a licensed smallpox vaccine (ACAM2000) based on a Plaque Reduction Neutralization Test (PRNT) using the Western Reserve strain of vaccinia virus and was supported by efficacy data from animal challenge studies. [see Nonclinical Toxicology (13.2)]
Vaccine effectiveness against mpox was inferred from the immunogenicity of JYNNEOS in a clinical study and from efficacy data from animal challenge studies.
[see Nonclinical Toxicology (13.2)]
14.2 Immunogenicity
Study 10 [10] (N=433) was a randomized, open-label study conducted at US military facilities in South Korea to compare the immunogenicity of JYNNEOS to ACAM2000 in healthy smallpox vaccine-naïve adults 18 through 42 years of age. Participants were randomized to receive either two doses of JYNNEOS (N=220) administered 28 days apart or one dose of ACAM2000 (N=213). In the total study population, the mean age was 24 years and 23 years in participants receiving JYNNEOS and ACAM2000, respectively; 82.3% and 86.4% of the participants were men; 57.3% and 63.8% were white/Caucasian, 21.8% and 18.8% black/African American, 6.4% and 5.6% Asian, 3.6% and 2.8% American Indian/Alaska Native, 2.3% and 1.4% Native Hawaiian/Other Pacific, 8.6% and 7.5% other racial groups, and 24.5% and 18.8% of Hispanic/Latino ethnicity (JYNNEOS and ACAM2000, respectively).
The primary immunogenicity endpoint was geometric mean titer (GMT) of vaccinia neutralizing antibodies assessed by PRNT at “peak visits” defined as two weeks after the second dose of JYNNEOS and four weeks after the single dose of ACAM2000. Analyses of antibody responses were performed in the per-protocol immunogenicity (PPI) population, consisting of participants who received all vaccinations and completed all visits up until two weeks after the second dose of JYNNEOS without major protocol violations pertaining to immunogenicity assessments. Table 3 presents the pre-vaccination and “peak visit” PRNT GMTs from Study 10.
| Time Point | JYNNEOSa (N=185)
GMTb [95% CI] | ACAM2000a (N=186)
GMTb [95% CI] |
|---|---|---|
| x NCT01913353 y Per Protocol Set for Immunogenicity included participants who received all vaccinations, completed all visits up until the specified “peak visits” (two weeks after the second dose of JYNNEOS or 4 weeks after the single dose of ACAM2000) without major protocol violations pertaining to immunogenicity assessments. a JYNNEOS was administered as a series of two doses given 28 days apart, and ACAM2000 was administered as a single dose. b GMT of vaccinia-neutralizing antibody titers assessed by plaque reduction neutralization test (PRNT) using the Western Reserve vaccinia strain. Values below the assay lower limit of quantitation (LLOQ) of 20 were imputed to a titer of 10; the proportions of participants with pre-vaccination titers less than the assay lower limit of detection were 98.9% among participants randomized to JYNNEOS and 97.8% among participants randomized to ACAM2000, respectively. c Non-inferiority of the “peak visit” PRNT GMT for JYNNEOS compared to ACAM2000 was demonstrated as the lower bound of the 1-sided 97.5% CI for the GMT ratio (JYNNEOS/ACAM2000) was > 0.5. N: Number of participants in the specified treatment group; GMT: Geometric Mean Titer; 95% CI: 95% confidence interval, lower limit and upper limit. |
||
|
Pre-Vaccination |
10.1 [9.9, 10.2] |
10.0 [10.0, 10.0] |
|
Post-Vaccination |
152.8c [133.3, 175.0] |
84.4c [73.4, 97.0] |
PRNT GMTs were also evaluated at pre-specified time points post-vaccination and prior to two weeks after the second dose of JYNNEOS. The PRNT GMTs at two and four weeks after the first dose of JYNNEOS (prior to the second dose), were 23.4 (95% CI: 20.5, 26.7) and 23.5 (95% CI: 20.6, 26.9), respectively. The PRNT GMT at two weeks after the single dose of ACAM2000 was 23.7 (95% CI: 20.9, 26.8).
Study 2 [2] was a randomized, double-blind, multicenter study conducted in the US to assess non-inferiority of immune responses induced by the two-vial presentation of JYNNEOS compared to immune responses induced by the one-vial presentation of JYNNEOS in healthy smallpox vaccine-naïve adults 18 through 55 years of age [see Adverse Reactions (6.1)]. In the Per Protocol Set (PPS; one-vial: N=297; two-vial: N=306), consisting of participants who received all vaccinations and completed all visits up until two weeks after the second dose of JYNNEOS without major protocol violations, the mean age was 28 years for both study groups; 52.9% of the participants in both study groups were women; 72.7% of the participants administered the one-vial presentation of JYNNEOS and 75.2% of the participants administered the two-vial presentation of JYNNEOS were White/Caucasian, 24.2% and 21.9% were Black/African American, respectively, 1.0% and 0% Oriental/Asian, 0% and 0.3% American Indian/Alaska Native, 0.3% and 0.3% Native Hawaiian/Other Pacific, 1.7% and 1.6% multiple racial groups, 0% and 0.7% other racial groups, and 27.3% and 27.5% of Hispanic/Latino ethnicity, respectively.
The primary immunogenicity endpoint was GMT of anti-vaccinia antibodies assessed by Enzyme-linked Immunosorbent Assay (ELISA), and a secondary immunogenicity endpoint was GMT of vaccinia-specific neutralizing antibodies assessed by PRNT, at two weeks after the second dose of JYNNEOS. Analyses of antibody responses were performed on the PPS. Table 4 presents the pre-and post-vaccination ELISA and PRNT GMTs from Study 2.
| x NCT01668537 y Per Protocol Set included participants who received all vaccinations, completed all visits without major protocol violations. a JYNNEOS was administered as a series of two doses given 4 weeks apart. b GMT of anti-vaccinia antibody titers assessed by Enzyme-linked Immunosorbent Assay (ELISA). Values below the assay lower limit of quantitation (LLOQ) of 100 were imputed to a titer of 50; the proportions of participants with prevaccination titers less than the assay lower limit of detection were 98.0% among participants randomized to one-vial JYNNEOS and 97.7% among participants randomized to two-vial JYNNEOS. c GMT of vaccinia-neutralizing antibody titers assessed by Plaque Reduction Neutralization Test (PRNT). Values below the assay LLOQ of 20 were imputed to a titer of 10; the proportions of participants with pre-vaccination titers less than the assay lower limit of detection were 98.0% among participants randomized to one-vial JYNNEOS and 98.7% among participants randomized to two-vial JYNNEOS. d Non-inferiority of the ELISA GMT for JYNNEOS was demonstrated as the upper bound of the 2-sided 95% CI for the GMT ratio (one-vial JYNNEOS/two-vial JYNNEOS) was below the margin of 1.5. N: Number of participants in the specified treatment group; GMT: Geometric Mean Titer; 95% CI: 95% confidence interval, lower limit and upper limit. |
||||
|
Time Point |
ELISA |
PRNT |
||
|
JYNNEOSa One-Vial Presentation (N=297) GMTb [95% CI] |
JYNNEOSa Two-Vial Presentation (N=306) GMTb [95% CI] |
JYNNEOSa One-Vial Presentation (N=297) GMTc [95% CI] |
JYNNEOSa Two-Vial Presentation (N=306) GMTc [95% CI] |
|
|
Pre-Vaccination |
50.5 [49.7, 51.2] |
50.3 [49.7, 51.0] |
10.2 [9.9, 10.4] |
10.1 [10.0, 10.3] |
|
Two Weeks Post Dose 2y |
875.1d [801.3, 955.7] |
1099.6d [1014.8, 1191.6] |
82.7 [74.3, 92.1] |
100.5 [90.3, 111.8] |
15. References
1. Study 1: NCT01144637
2. Study 2: NCT01668537
3. Study 3: NCT00316524
4. Study 4: NCT00686582
5. Study 5: NCT00857493
6. Study 6: NCT00316589
7. Study 7: NCT00189904
8. Study 8: NCT00316602
9. Study 9: NCT00189917
10. Study 10: NCT01913353
16. How is Jynneos supplied
16.1 JYNNEOS One-Vial Presentation
How Supplied
JYNNEOS one-vial presentation is supplied in cartons containing either:
- •
- 10 single-dose vials of JYNNEOS (yellow cap), or
- •
- 20 single-dose vials of JYNNEOS (yellow cap)
|
Carton |
Carton NDC Number |
Vial NDC Number |
|
Carton of 10 vials |
50632-023-02 |
50632-023-04 |
|
Carton of 20 vials |
50632-022-02 |
50632-022-04 |
Storage Conditions
Keep frozen at -25°C to -15°C (-13°F to +5°F).
Store in the original package to protect from light.
Do not re-freeze a vial once it has been thawed.
Once thawed, the vaccine may be kept at +2°C to +8°C (+36°F to +46°F) for 4 weeks.
Do not use the vaccine after the expiration.
16.2 JYNNEOS Two-Vial Presentation
How Supplied
JYNNEOS two-vial presentation is supplied in separate cartons:
- •
- 20 vials of Lyophilized Antigen Component (Vial A, yellow cap)
- •
- 20 vials of Diluent Component (Vial B, blue cap)
To form a single dose of JYNNEOS, reconstitute one vial of Lyophilized Antigen Component (Vial A, yellow cap) with one vial of Diluent Component (Vial B, blue cap).
|
Carton |
Carton NDC Number |
Vial NDC Number |
|
Carton of 20 vials of Lyophilized Antigen Component |
50632-002-02 |
50632-002-01 |
|
Carton of 20 vials of Diluent Component |
50632-003-02 |
50632-003-01 |
Storage Before Reconstitution
- •
- Lyophilized Antigen Component:
- Keep FROZEN at -25°C to -15°C (-13°F to +5°F).
- Store in the original package to protect vial from light.
- Do not use the vaccine after the expiration date shown on the vial label.
- •
- Diluent Component:
- Store at room temperature (+20°C to +25°C, +68°F to +77°F) or refrigerated (+2°C to +8°C, +36°F to +46°F).
Storage After Reconstitution
After reconstitution, use immediately or store refrigerated at +2°C to +8°C (+36°F to +46°F), away from light, for up to 12 hours. Do not freeze reconstituted vaccine.
17. Patient Counseling Information
- •
- Inform vaccine recipient of the potential benefits and risks of vaccination with JYNNEOS.
- •
- Inform vaccine recipient of the importance of completing the two dose vaccination series.
- •
- Advise vaccine recipient to report any adverse events to their healthcare provider or to the Vaccine Adverse Event Reporting System at 1-800-822-7967 and www.vaers.hhs.gov.
Manufactured by:
Bavarian Nordic A/S
Philip Heymans Alle 3
2900 Hellerup
Denmark
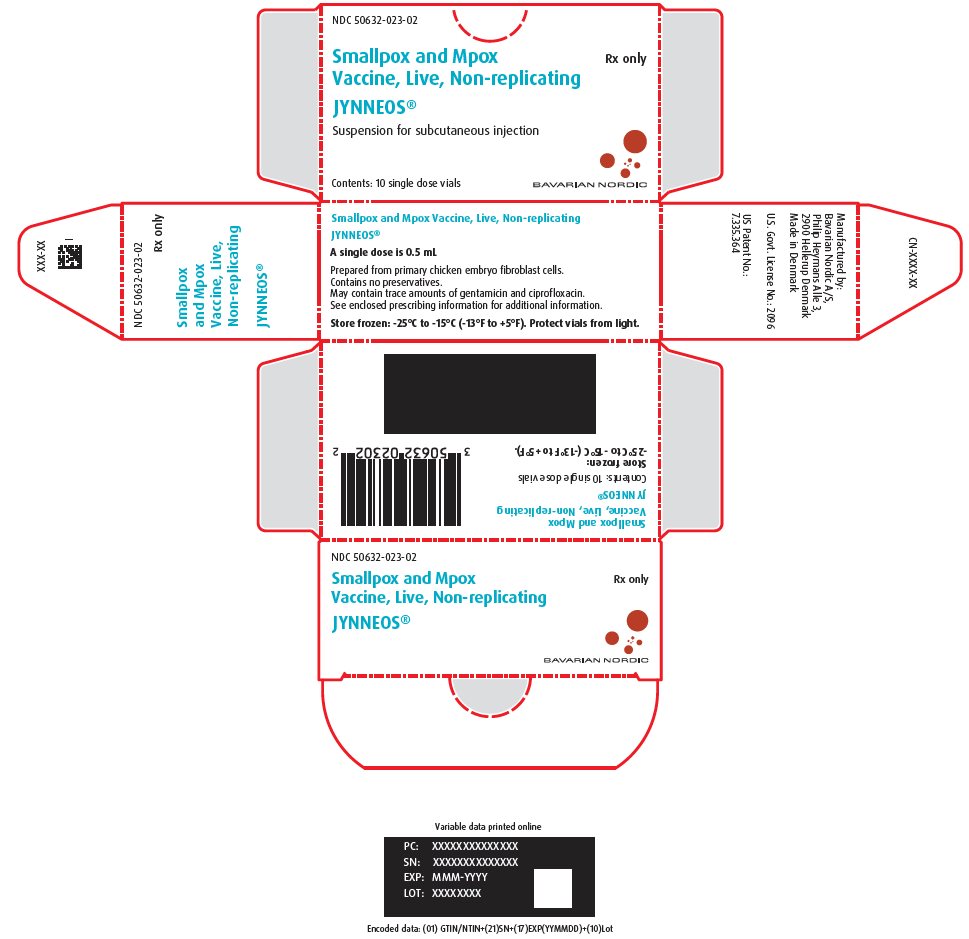
PRINCIPAL DISPLAY PANEL – One-Vial Presentation Carton Label – 10 vials
NDC 50632-023-02
Smallpox and Mpox Rx only
Vaccine, Live, Non-replicating
JYNNEOS®
Suspension for subcutaneous injection
Contents: 10 single dose vials
BAVARIAN NORDIC
PRINCIPAL DISPLAY PANEL – One-Vial Presentation Carton Label – 20 vials
NDC 50632-022-02
Smallpox and Mpox Rx only
Vaccine, Live, Non-replicating
JYNNEOS®
Suspension for subcutaneous injection
Contains 20 single-dose 0.5 mL vials
BAVARIAN NORDIC
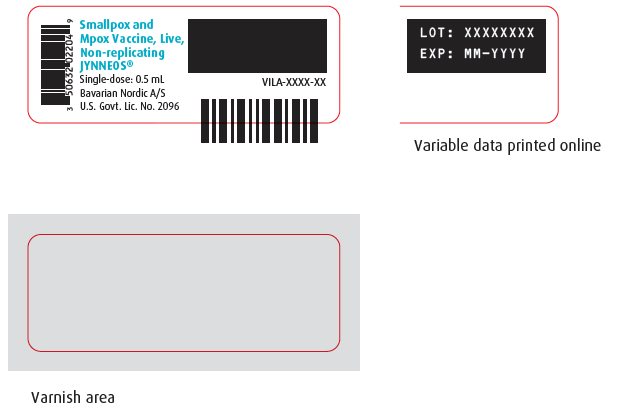
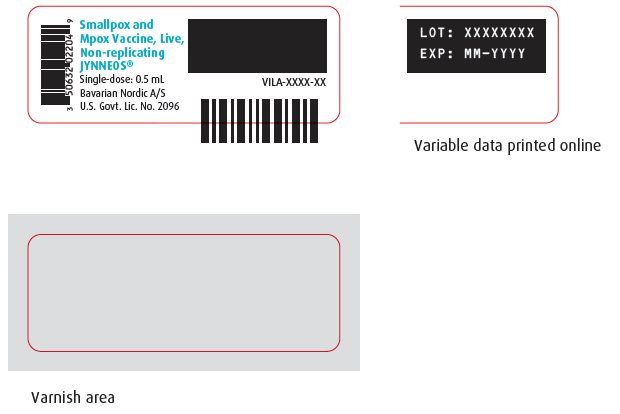
PRINCIPAL DISPLAY PANEL – One-Vial Presentation Vial Labels
Vial Label – NDC 50632-023-04
Vial Label - NDC 50632-022-04
Smallpox and
Mpox Vaccine, Live,
Non-replicating
JYNNEOS®
Single-dose: 0.5 mL
Bavarian Nordic A/S
U.S. Govt. Lic. No. 2096
VILA-XXXX-XX
LOT: XXXXXXXX
EXP: MM-YYYY
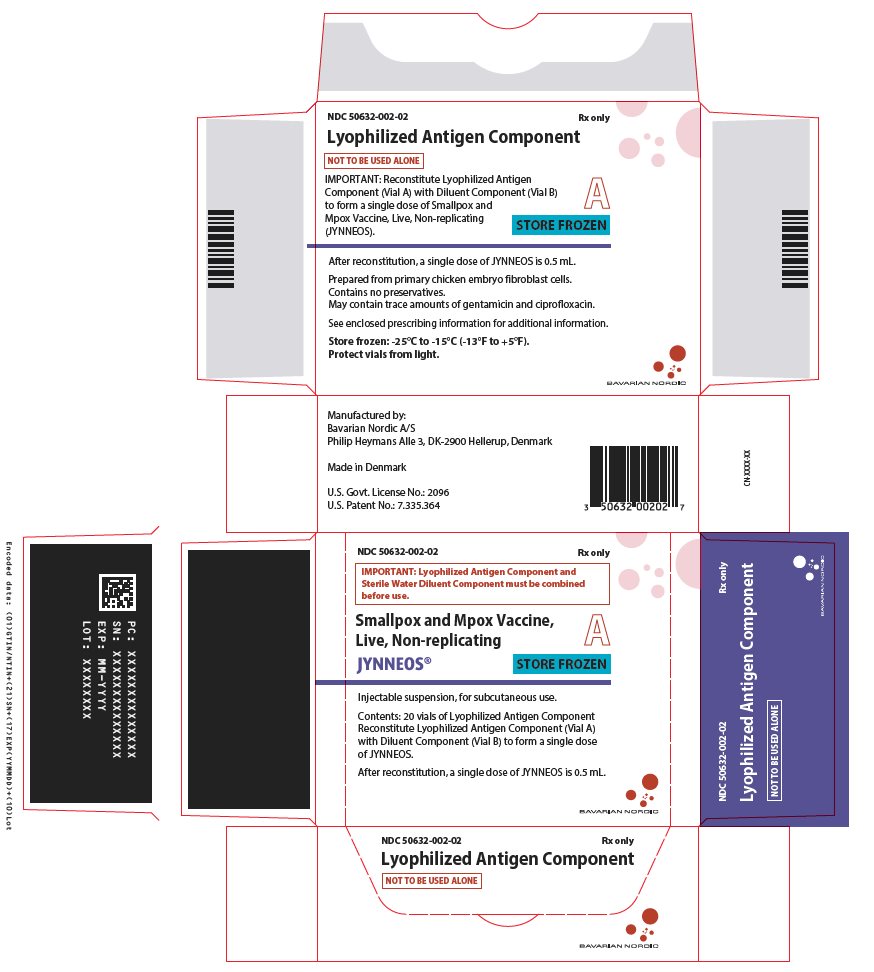
PRINCIPAL DISPLAY PANEL – Two-Vial Presentation Antigen Carton Label
NDC 50632-002-02 Rx only
IMPORTANT: Lyophilized Antigen Component and
Sterile Water Diluent Component must be combined
before use.
Smallpox and Mpox Vaccine,
Live, Non-replicating
JYNNEOS®
A
STORE FROZEN
Injectable suspension, for subcutaneous use.
Contents: 20 vials of Lyophilized Antigen Component
Reconstitute Lyophilized Antigen Component (Vial A)
with Diluent Component (Vial B) to form a single dose
of JYNNEOS.
After reconstitution, a single dose of JYNNEOS is 0.5 mL.
BAVARIAN NORDIC
PRINCIPAL DISPLAY PANEL - Two-Vial Presentation Antigen Vial Label
NDC 50632-002-01 Rx only
Lyophilized Antigen
Component
NOT TO BE USED ALONE
A
Reconstitute with Diluent Component
to form Smallpox and Mox Vaccine
Live, Non-replicating (JYNNEOS).
After reconstitution, a single dose of
JYNNEOS is 0.5 mL
LOT:
EXP:
Mfd. by Bavarian Nordic A/S
VILA-XXXX-XX
PRINCIPAL DISPLAY PANEL - Two-Vial Presentation Diluent Carton Label
NDC 50632-003-02 Rx only
Diluent Component
NOT TO BE USED ALONE
B
Use Diluent Component (Vial B) to reconstitute Lyophilized
Antigen Component (Vial A) to form a single dose of Smallpox
and Mpox Vaccine, Live, Non-replicating (JYNNEOS).
Contents: 20 vials of Diluent Component.
Contains no preservative.
See prescribing information is accompanying Lyophilized
Antigen Component Package A for additional information.
BAVARIAN NORDIC
PRINCIPAL DISPLAY PANEL - Two-Vial Presentation Diluent Vial Label
NDC 50632-003-01 Rx only
Diluent Component
NOT TO BE USED ALONE
B
Use to reconstitute Lyophilized
Antigen Component (Vial A) to form
Smallpox and Mpox Vaccine, Live,
Non-replicating (JYNNEOS).
Mfg. by Bavarian Nordic A/S.
LOT:
EXP:
Mfd. by Bavarian Nordic A/S
VILA-XXXX-XX
| JYNNEOS
vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| JYNNEOS
vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| JYNNEOS
vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen injection, powder, lyophilized, for suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DILUENT COMPONENT
water for injection liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bavarian Nordic A/S (310209754) |
More about Jynneos (smallpox and mpox vaccine)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: viral vaccines
- En español