ACAM2000: Package Insert / Prescribing Info
Package insert / product label
Generic name: smallpox (vaccinia) vaccine, live
Dosage form: injection, powder, lyophilized, for solution
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Mar 30, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
ACAM2000® [Smallpox and Mpox (Vaccinia) Vaccine, Live]
For scarification suspension, for percutaneous use
Initial U.S. Approval: 2007
WARNING: SERIOUS COMPLICATIONS
See full prescribing information for complete boxed warning.
Myocarditis and pericarditis (suspect cases observed at a rate of 5.7 per 1000 primary vaccinees (95% CI: 1.9-13.3)), encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including STEVENS-JOHNSON SYNDROME), eczema vaccinatum resulting in permanent sequelae or death, accidental eye infection (ocular vaccinia) which can cause ocular complications that may lead to blindness, and fetal death, have occurred following either primary vaccination or revaccination with ACAM2000 or other live vaccinia virus vaccines that were used historically. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequelae and/or death [see Warnings and Precautions (5)].
Recent Major Changes
Indications and Usage, mpox disease (1) 8/2024
Indications and Usage for ACAM2000
ACAM2000® is indicated for active immunization for the prevention of smallpox and mpox disease in individuals determined to be at high risk for smallpox or mpox infection. (1).
ACAM2000 Dosage and Administration
For percutaneous use by scarification. (2)
- •
- Administer ACAM2000 only after being trained on the safe and effective administration of the vaccine. (2.4)
- •
- A droplet of ACAM2000 is administered by scarification percutaneously using 15 jabs of a bifurcated needle. (2.4)
- •
- The droplet (approximately 0.0025 mL) of reconstituted vaccine is picked up with a bifurcated needle by dipping needle into ACAM2000 vial. (2.4)
- •
- See full prescribing information for instructions for vaccine preparation (2.2), administration including provision of the Medication Guide to vaccinees and instruction to vaccinees about vaccination site care (2.6) and interpretation of response to vaccination. (2.7)
Dosage Forms and Strengths
Contraindications
- •
- Individuals with severe immunodeficiency. These individuals may include persons who are undergoing bone marrow transplantation or persons with primary or acquired immunodeficiency states who require isolation. (4).
Warnings and Precautions
- •
- Myocarditis and/or pericarditis, ischemic heart disease and non-ischemic dilated cardiomyopathy. (5.1, 5.2)
- •
- Encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia (vaccinia necrosum), generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson syndrome), eczema vaccinatum, fetal vaccinia and fetal death. (5.1)
- •
- Ocular vaccinia that may lead to blindness. (5.3)
- •
- These risks, including risks of severe disability and/or death, are increased in vaccinees with:
- •
- ACAM2000 is a live vaccinia virus that can be transmitted to persons who have close contact with the vaccinee and the risks in contacts are the same as those stated for vaccinees. (5.10)
Adverse Reactions/Side Effects
Common adverse reactions include inoculation site signs and symptoms, lymphadenitis, and constitutional symptoms, such as malaise, fatigue, fever, myalgia, and headache (6). These adverse reactions are less frequent in revaccinated persons than persons receiving the vaccine for the first time.
Inadvertent inoculation at other sites is the most frequent complication of vaccinia vaccination. The most common sites involved are the face, nose, mouth, lips, genitalia and anus.
Self-limited skin rashes not associated with vaccinia replication in skin, including urticaria and folliculitis, may occur following vaccination.
To report SUSPECTED ADVERSE REACTIONS, contact Emergent BioSolutions at 1-877-246-8472 and medicalinformation@ebsi.com or VAERS at 1-800-822-7967 and https://vaers.hhs.gov.
Use In Specific Populations
- •
- ACAM2000 may rarely cause fetal infection, usually resulting in stillbirth or death. (8.1)
- •
- ACAM2000 live vaccinia virus may be transmitted from a lactating mother to her infant causing complications in the infant from inadvertent inoculation. (8.2)
- •
- ACAM2000 may be associated with an increased risk of serious complications in children, especially in infants younger than 12 months. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2025
Full Prescribing Information
WARNING: SERIOUS COMPLICATIONS
- •
- Suspected cases of myocarditis and/or pericarditis have been observed in healthy adult primary vaccinees (at an approximate rate of 5.7 per 1000, 95% CI: 1.9-13.3) receiving ACAM2000 [see Warnings and Precautions (5.1)].
- •
- Encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including STEVENS-JOHNSON SYNDROME), eczema vaccinatum resulting in permanent sequelae or death, and fetal death have occurred following either primary vaccination or revaccination with ACAM2000 or other live vaccinia virus vaccines that were used historically [see Warnings and Precautions (5)].
- •
- Accidental eye infection (ocular vaccinia) may result in ocular complications including keratitis and corneal scarring that may lead to blindness [see Warning and Precautions (5.3)].
- •
-
These risks may result in severe disability, permanent neurological sequelae and/or death and are increased in individuals who:
- •
- Have cardiac disease or a history of cardiac disease
- •
- Have eye disease treated with topical steroids
- •
- Have congenital or acquired immune deficiency disorders, including individuals taking immunosuppressive medications
- •
- Have eczema or a history of eczema or other acute or chronic exfoliative skin conditions
- •
- Are less than 12 months of age
- •
- Are pregnant
ACAM2000 contains live vaccinia virus that can be transmitted to persons who have close contact with the vaccinee and the risks in contacts are the same as those for the vaccinee.
The risk for experiencing serious vaccination complications must be weighed against the risks for experiencing a potentially severe or fatal smallpox or mpox infection.
1. Indications and Usage for ACAM2000
ACAM2000® is a vaccine indicated for active immunization for the prevention of smallpox and mpox disease in individuals determined to be at high risk for smallpox or mpox infection.
2. ACAM2000 Dosage and Administration
2.1 Route of Administration
Administer ACAM2000 only after being trained on the safe and effective administration of the vaccine by the percutaneous route (scarification).
Do not administer ACAM2000 by injection.
2.2 Vaccine Preparation
ACAM2000 is supplied as a multiple dose vial of Lyophilized Antigen Component, Live to be reconstituted with the supplied Diluent Component.
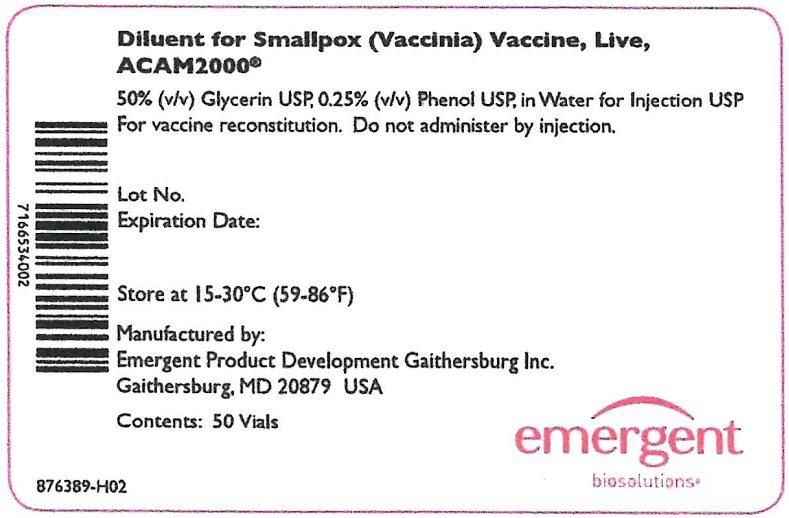
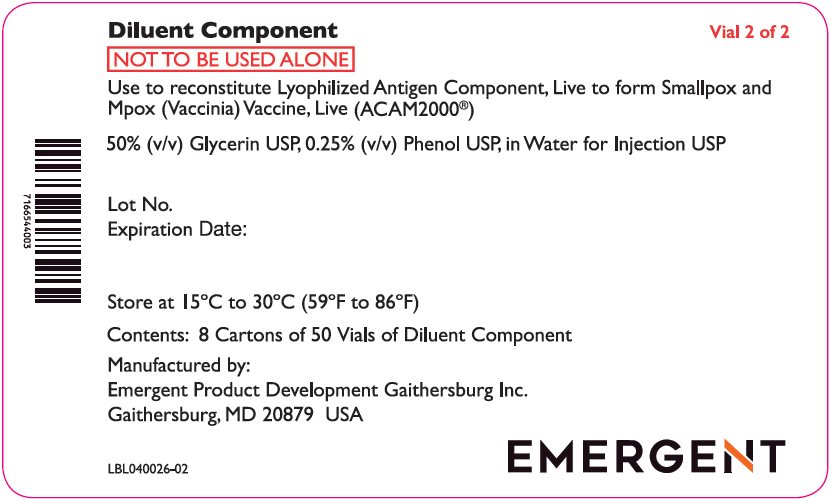
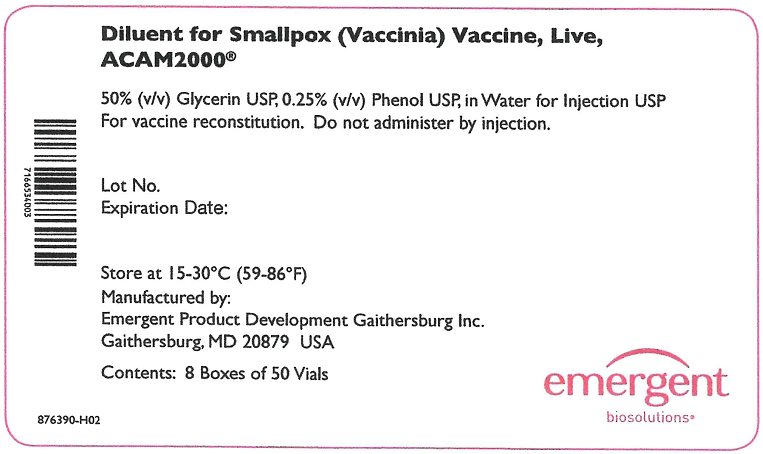
The Lyophilized Antigen Component, Live is supplied in cartons and vials labeled “Smallpox (Vaccinia) Vaccine, Live, ACAM2000.” The Diluent Component may be supplied in cartons and vials labeled “Diluent for Smallpox (Vaccinia) Vaccine, Live, ACAM2000.” Reconstitution instructions and storage following reconstitution are described below.
Reconstitution
Reconstitute the Lyophilized Antigen Component, Live only with 0.3 mL of the provided Diluent Component to form ACAM2000. Note: 0.3 mL of diluent is not the entire content of the vial of Diluent Component.
Remove the Lyophilized Antigen Component, Live vial from freezer or refrigerator and bring to room temperature before reconstitution.
Remove the flip cap seals of the Lyophilized Antigen Component, Live and Diluent Component vials and wipe with an isopropyl alcohol swab and allow to dry thoroughly.
Use aseptic technique and a sterile 1 mL syringe fitted with a 25 gauge x 5/8” needle (provided) to draw up 0.3 mL from the Diluent Component vial and transfer the entire content of the syringe to the Lyophilized Antigen Component, Live vial.
Gently swirl to mix, do not shake or invert the vial, and avoid product contact with the rubber stopper.
The reconstituted vaccine should be a clear to slightly hazy, colorless to straw-colored liquid free from extraneous matter. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter or discoloration is observed, do not use the vaccine and dispose of the vial as biohazardous material [see Dosage and Administration (2.5)].
After reconstitution, each vial contains 100 nominal doses of approximately 0.0025 mL each.
Storage following Reconstitution
After reconstitution, ACAM2000 may be administered within 8 hours if kept at 20°C to 25°C (68°F to 77°F). Store unused, reconstituted ACAM2000 in a refrigerator 2°C to 8°C (36°F to 46°F) for up to 30 days, after which the unused portion is to be discarded as a biohazardous material [see Dosage and Administration (2.5)]. Minimize exposure of reconstituted vaccine to room temperature during vaccination sessions by placing it in the refrigerator or on ice between patient administrations.
2.3 Preparation / Handling Precautions
Wear surgical or protective gloves when preparing and administering the vaccine to avoid contact of vaccine with skin, eyes or mucous membranes.
2.4 Vaccine Administration
Bring the reconstituted vaccine to room temperature prior to administration. Before administration, examine the vial contents to verify the absence of particulates and gently swirl, without allowing the product to contact the rubber stopper, if necessary to re-dissolve any precipitates that might have formed.
The site of vaccination is the upper arm over the insertion of the deltoid muscle.
No skin preparation should be performed unless the skin at the intended site of vaccination is obviously dirty, in which case an alcohol swab(s) may be used to clean the area. If alcohol is used, the skin must be allowed to dry thoroughly to prevent inactivation of the live vaccine virus by the alcohol.
Remove the vaccine vial cap. Remove bifurcated needle from individual wrapping. Submerge bifurcated end of needle in reconstituted vaccine solution. The needle will pick up a droplet of vaccine (approximately 0.0025 mL) within the fork of the bifurcation. Use aseptic technique, i.e., do not insert the upper part of the needle that has been in contact with fingers into the vaccine vial, and never redip the needle into the vaccine vial if the needle has touched skin.
Deposit the droplet of vaccine onto clean, dry skin of the arm prepared for vaccination. The needle is held between thumb and first finger perpendicular to the skin. The wrist of the vaccinator’s hand holding the needle rests against the vaccine recipient’s arm. Rapidly make 15 jabs of the needle perpendicular to the skin through the vaccine droplet to puncture the skin, within a diameter of about 5 mm. The jabs should be vigorous enough so that a drop of blood appears at the vaccination site.
Wipe any excess droplets of vaccine and blood off the skin using a dry gauze pad and discard in a biohazard container. Discard the needle in a biohazard sharps container [see Dosage and Administration (2.5)]. Close the vaccine vial by reinserting the rubber cap and return to a refrigerator or place on ice unless it will be used immediately to vaccinate another individual.
2.5 Disposal
Discard the Lyophilized Antigen Component, Live vial (including any unused reconstituted vaccine), its stopper, the syringe and needle used for reconstitution, the bifurcated needle used for administration, and any gauze or cotton that came in contact with the vaccine in leak-proof, puncture-proof biohazard containers. Dispose of these containers appropriately.
2.6 Care of the Vaccination Site
Ensure each vaccine recipient has the FDA-approved Medication Guide, which includes instructions for care of the vaccination site.
Cover vaccination site with gauze and secure it loosely with first aid adhesive tape. The gauze provides a barrier to protect against spread of the vaccinia virus. If the vaccinee is involved in direct patient care, the gauze may be covered with a semipermeable (semi-occlusive) dressing as an additional barrier. A semipermeable dressing is one that allows for the passage of air but does not allow for the passage of fluids.
Do not use a bandage that blocks air from the vaccination site. This may cause the skin at the vaccination site to soften and wear away.
Wash hands with soap and warm water or with alcohol-based hand rubs such as gels or foams after direct contact with the vaccination site, the gauze and semipermeable dressing, clothes, towels or sheets that might be contaminated with virus from the vaccination site. This is vital in order to remove any virus from your hands and prevent contact spread.
Throw away the contaminated gauze and semipermeable dressing in a sealed plastic bag. A small amount of bleach can be added to the bag to kill the vaccine virus.
Wash separately clothing, towels, bedding or other items that may have come in direct contact with the vaccination site or drainage from the site, using hot water with detergent and/or bleach. Wash hands afterwards.
Do not put salves or ointments on the vaccination site.
2.7 Interpreting Vaccination Response
Primary Vaccinees
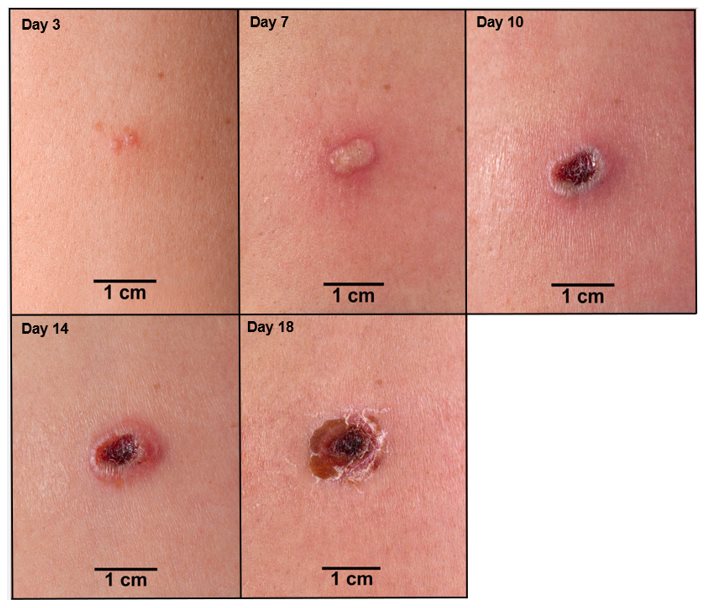
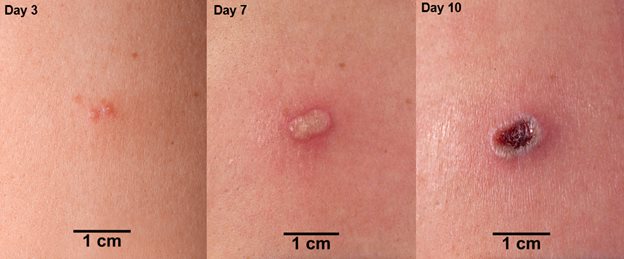
In an individual vaccinated for the first time (primary vaccination; vaccinia-naïve), the expected response to vaccination is the development of a major cutaneous reaction (characterized by a pustule) at the site of inoculation. The lesion evolves gradually, with appearance of a papule at the site of vaccination after 2-5 days. The papule becomes vesicular surrounded by a red areola, then pustular, and reaches its maximum size at 8-10 days after vaccination; the pustule dries and forms a scab (See Figure 1). In primary vaccinees, scab separation and re-epithelialization occurs 3-6 weeks after vaccination, leaving a pitted scar. Formation of a major cutaneous reaction by day 6-11 is evidence of a successful ‘take’ and acquisition of protective immunity. An equivocal reaction is any reaction that is not a major reaction and indicates a non-take (vaccination failure) due to impotent vaccine or inadequate vaccination technique.
Individuals who are not successfully vaccinated (i.e., vaccination failures) after primary vaccination may be revaccinated again in an attempt to achieve a satisfactory take. The vaccination procedures should be checked, and vaccination repeated with vaccine from another vial or vaccine lot, employing the same technique described in section 2.4 [see Dosage and Administration (2.4)]. If a repeat vaccination is conducted using vaccine from another vial or vaccine lot fails to produce a major reaction, healthcare providers should consult the Centers for Disease Control and Prevention (CDC) Emergency Operations Center (EOC) at 770-488-7100 and their state or local health department before giving another vaccination.
Previously Vaccinated Individuals (Revaccination)
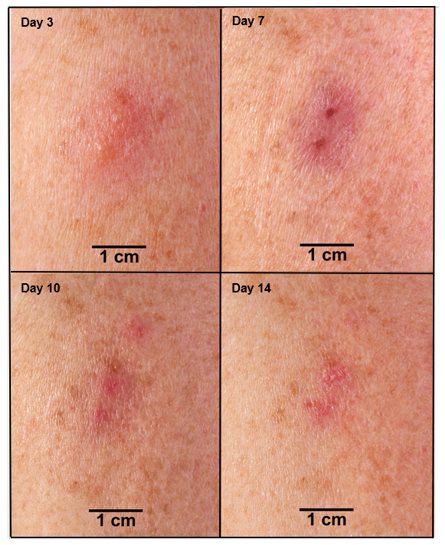
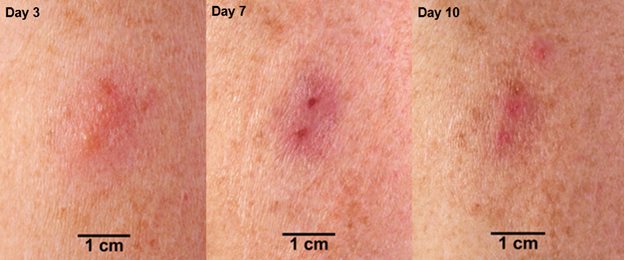
Based on data from Study 2 [see Adverse Reactions (6.1)], successful vaccination in an individual previously exposed to vaccine (vaccinia-experienced) is confirmed when a major cutaneous reaction is observed 6 to 8 days post-vaccination. A major cutaneous reaction at the vaccination site heals through the same stages as described in primary vaccinees and scab separation may occur earlier in revaccinated individuals than in primary vaccinees (Figure 2). However, any prior vaccination may modify (reduce) the cutaneous response upon revaccination (Figure 2) such that the absence of a cutaneous response does not necessarily indicate vaccination failure. Vaccinia-experienced individuals who do not have a cutaneous response on revaccination do not require revaccination to try to elicit a cutaneous response.
Figure 1 Progression of Major Cutaneous Reaction After Primary Vaccination (1)
Figure 2 Progression of Major Cutaneous Reaction After Revaccination (1)
3. Dosage Forms and Strengths
For scarification suspension. ACAM2000 is supplied as a multiple dose vial of Lyophilized Antigen Component, Live to be reconstituted with the supplied Diluent Component. After reconstitution, a single dose of ACAM2000 is approximately 0.0025 mL.
4. Contraindications
Severe Immune Deficiency
Do not administer ACAM2000 to individuals with severe immunodeficiency. These individuals may include individuals who are undergoing bone marrow transplantation or individuals with primary or acquired immunodeficiency who require isolation.
5. Warnings and Precautions
5.1 Serious Complications
Serious complications that may follow either primary or revaccination with ACAM2000 include: myocarditis and/or pericarditis, encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia (vaccinia necrosum), generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson syndrome), eczema vaccinatum, accidental eye infection (ocular vaccinia) which can cause ocular complications including keratitis and corneal scarring that may lead to blindness, and fetal death in pregnant women. These complications may rarely lead to severe disability, permanent neurological sequalae and death. Based on ACAM2000 clinical trials, symptoms of suspected myocarditis or pericarditis (such as chest pain, raised troponin/cardiac enzymes, or ECG abnormalities) occurred in 5.7 per 1000 primary vaccinations. This finding includes cases of acute symptomatic or asymptomatic myocarditis or pericarditis or both. Historically, death following vaccination with live vaccinia virus is a rare event; approximately 1 death per million primary vaccinations and 1 death per 4 million revaccinations have occurred after vaccination with live vaccinia virus. Death is most often the result of sudden cardiac death, post-vaccinial encephalitis, progressive vaccinia, or eczema vaccinatum. Death has also been reported in unvaccinated contacts accidentally infected by individuals who have been vaccinated.
Incidence of Serious Complications in 1968 U.S. Surveillance Studies
Estimates of the risks of occurrence of serious complications after primary vaccination and revaccination, based on safety surveillance studies conducted when live vaccinia virus smallpox vaccine (i.e., New York City Board of Health strain, Dryvax) was routinely recommended, are as follows:
| Age (yrs) | <1 | 1-4 | 5-19 | ≥20 | Overall rates(h) |
|---|---|---|---|---|---|
| a. See article for descriptions of complications. | |||||
| b. Adapted from (3) and (4). | |||||
| c. Referenced as accidental implantation. | |||||
| d. Referenced as vaccinia necrosum. | |||||
| e. Death from all complications. | |||||
| f. Rates of overall complications by age group include complications not provided in this table, including severe local reactions, bacterial superinfection of the vaccination site, and erythema multiforme. | |||||
| g. No instances of this complication were identified during the 1968 ten (10) state survey. | |||||
| h. Overall rates for each complication include persons of unknown age. | |||||
|
Inadvertent inoculation(c) |
507.0 |
577.3 |
371.2 |
606.1 |
529.2 |
|
Generalized vaccinia |
394.4 |
233.4 |
139.7 |
212.1 |
241.5 |
|
Eczema vaccinatum |
14.1 |
44.2 |
34.9 |
30.3 |
38.5 |
|
Progressive vaccinia(d) |
--(g) |
3.2 |
--(g) |
--(g) |
1.5 |
|
Postvaccinial encephalitis |
42.3 |
9.5 |
8.7 |
--(g) |
12.3 |
|
Death(e) |
5 |
0.5 |
0.5 |
unknown |
-- |
|
Total(f) |
1549.3 |
1261.8 |
855.9 |
1515.2 |
1253.8 |
| Age (yrs) | <1 | 1-4 | 5-19 | ≥20 | Overall rates(b) |
|---|---|---|---|---|---|
| See Table 1 for explanation of footnotes. | |||||
|
Inadvertent inoculation(c) |
(g) |
109.1 |
47.7 |
25.0 |
42.1 |
|
Generalized vaccinia |
(g) |
(g) |
9.9 |
9.1 |
9.0 |
|
Eczema vaccinatum |
(g) |
(g) |
2.0 |
4.5 |
3.0 |
|
Progressive vaccinia(d) |
(g) |
(g) |
(g) |
6.8 |
3.0 |
|
Postvaccinial encephalitis |
(g) |
(g) |
(g) |
4.5 |
2.0 |
|
Death(e) |
-- |
-- |
-- |
-- |
-- |
|
Total(f) |
(g) |
200.0 |
85.5 |
113.6 |
108.2 |
Incidence of Serious Complications and Emergence of Myocarditis and/or Pericarditis in 2002-2005
Data on the incidence of adverse events among U.S. military personnel and civilian first responders vaccinated with Dryvax, a formerly licensed live vaccinia virus smallpox vaccine, during vaccination programs initiated in December 2002 are shown below in Table 3.
The incidence of preventable adverse events (eczema vaccinatum, contact transmission, and auto-inoculation) were notably lower in these programs when compared with data collected in the 1960s; presumably because of better vaccination screening procedures and routine use of protective bandages over the inoculation site. Myocarditis and pericarditis were not commonly reported following smallpox vaccination in the 1960s but emerged as a more frequent event based on more active surveillance in the military and civilian programs.
| Adverse event | Na | Incidence/ million | Nb | Incidence/million |
|---|---|---|---|---|
| a. Department of Defense program (n=730,580) as of Jan05 where 71% primary vaccination; 89% male; median age 28.5 years | ||||
| b. Department of Health and Human Services program (n=40, 422) as of Jan04 where 36% primary vaccination; 36% male; median age 47.1 years | ||||
|
Myo/pericarditis |
86 |
117.71 |
21 |
519.52 |
|
Post-vaccinial encephalitis |
1 |
1.37 |
1 |
24.74 |
|
Eczema vaccinatum |
0 |
0.00 |
0 |
0.00 |
|
Generalized vaccinia |
43 |
58.86 |
3 |
74.22 |
|
Progressive vaccinia |
0 |
0.00 |
0 |
0.00 |
|
Fetal vaccinia |
0 |
0.00 |
0 |
0.00 |
|
Contact transmission |
52 |
71.18 |
0 |
0.00 |
|
Auto-inoculation (non-ocular) |
62 |
84.86 |
20 |
494.78 |
|
Ocular vaccinia |
16 |
21.90 |
3 |
74.22 |
Myocarditis and Pericarditis in the ACAM2000 Clinical Trial Experience
In clinical trials involving 2983 subjects who received ACAM2000 and 868 subjects who received Dryvax, ten (10) cases of suspected myocarditis [0.2% (7 of 2983) ACAM2000 subjects and 0.3% (3 of 868) Dryvax subjects] were identified. The mean time to onset of suspected myocarditis and/or pericarditis from vaccination was 11 days, with a range of 9 to 20 days. All subjects who experienced these cardiac events were naïve to vaccinia. Of the 10 subjects, 2 were hospitalized. None of the remaining 8 cases required hospitalization or treatment with medication. Of the 10 cases, 8 were sub-clinical and were detected only by ECG abnormalities with or without associated elevations of cardiac troponin I. All cases were resolved by 9 months, with the exception of one female subject in the Dryvax group, who had persistent borderline abnormal left ventricular ejection fraction on echocardiogram. The best estimate of risk for myocarditis and pericarditis was derived from the Phase 3 ACAM2000 clinical trials where there was active monitoring for the potential of myocarditis and pericarditis. Among vaccinees naïve to vaccinia, 8 cases of suspected myocarditis and pericarditis were identified across both vaccine groups, for a total incidence rate of 6.9 per 1000 vaccinees (8 of 1,162). The incidence rate for the ACAM2000 vaccine group was 5.7 (95% CI: 1.9-13.3) per 1000 vaccinees (5 of 873 vaccinees) and for the Dryvax group was 10.4 (95% CI: 2.1-30.0) per 1000 vaccinees (3 of 289 vaccinees). No cases of myocarditis and/or pericarditis were identified in 1819 vaccinia-experienced subjects. The long-term outcome of myocarditis and pericarditis following ACAM2000 vaccination is currently unknown.
5.2 Cardiac Disease
Information on risks of myocarditis and pericarditis is presented in Section 5.1 [see Warnings and Precautions (5.1)].
Ischemic cardiac events, including fatal events, and non-ischemic, dilated cardiomyopathy have been reported following ACAM2000 and other live vaccinia virus vaccines that were used historically. The relationship of these events to vaccination is unknown.
There may be increased risks of adverse events with ACAM2000 in persons with known cardiac disease, including those diagnosed with previous myocardial infarction, angina, congestive heart failure, cardiomyopathy, chest pain or shortness of breath with activity, stroke or transient ischemic attack, or other heart conditions. In addition, individuals who have been diagnosed with 3 or more of the following risk factors for ischemic coronary disease may have increased risks: 1) high blood pressure; 2) elevated blood cholesterol; 3) diabetes mellitus or high blood sugar; 4) first degree relative (for example mother, father, brother, or sister) who had a heart condition before the age of 50; or 5) smoke cigarettes.
5.3 Ocular Complications
Accidental infection of the eye (ocular vaccinia) may result in ocular complications, including keratitis and corneal scarring that may lead to blindness. Patients who are using corticosteroid eye drops may be at increased risk of ocular complications with ACAM2000.
5.4 Presence of Congenital or Acquired Immune Deficiency Disorders
Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems, including patients with leukemia, lymphoma, organ transplantation, generalized malignancy, HIV/AIDS, cellular or humoral immune deficiency, radiation therapy, or treatment with antimetabolites, alkylating agents, high-dose corticosteroids (>10 mg prednisone/day or equivalent for ≥2 weeks), or other immunomodulatory drugs. ACAM2000 is contraindicated in individuals with severe immunodeficiency [see Contraindications (4)]. Close contacts of vaccinees (including sexual contacts) who have these conditions may be at increased risk because live vaccinia virus can be shed and be transmitted to close contacts.
5.5 History or Presence of Eczema and Other Skin Conditions
Persons with eczema of any description such as: atopic dermatitis, neurodermatitis, and other eczematous conditions, regardless of severity of the condition, or persons who have a history of these conditions at any time in the past, are at higher risk of developing eczema vaccinatum. Vaccinees with close contacts who have eczematous conditions, may be at increased risk because live vaccinia virus can shed and be transmitted to these close contacts. Vaccinees with other active acute, chronic or exfoliative skin disorders (including burns, impetigo, varicella zoster, acne vulgaris with open lesions, Darier’s disease, psoriasis, seborrheic dermatitis, erythroderma, pustular dermatitis, etc.), or vaccinees with household contacts having such skin disorders might also be at higher risk for eczema vaccinatum.
5.6 Infants < 12 months of Age
Based on data from historical use of other live vaccinia virus vaccines, the risk of serious adverse reactions following vaccination with ACAM2000 is higher in infants (<12 months of age). Vaccinated persons who have close contact with infants must take precautions to avoid inadvertent transmission of ACAM2000 live vaccinia virus to infants.
5.7 Pregnancy
ACAM2000 has not been studied in pregnant women. Based on data from historical use of other live vaccinia virus vaccines, ACAM2000 can cause fetal vaccinia and fetal death. If ACAM2000 is administered during pregnancy or within 6 weeks before becoming pregnant, the vaccinee should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)]. Vaccinees should be counseled to avoid becoming pregnant (or getting their partner pregnant) for 6 weeks after vaccination and until the vaccination site has healed.
Pregnant women who are close contacts of vaccinees may be at risk of adverse fetal outcomes because ACAM2000 live vaccinia virus can be transmitted from vaccinees. [see Use in Specific Populations (8.1)].
5.8 Severe Allergic Reactions
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of ACAM2000.
Persons who experienced a severe allergic reaction following a previous dose of ACAM2000 or following exposure to any ingredient of ACAM2000, including neomycin or polymyxin B, may be at increased risk for severe allergic reactions.
5.9 Management of ACAM2000 Complications
The CDC can assist physicians in the diagnosis and management of patients with suspected complications of ACAM2000 vaccination. Vaccinia immune globulin intravenous (human) (CNJ-016®) is indicated for the treatment of certain complications due to vaccinia vaccination. If CNJ-016 and/or other antivirals are needed or additional information is required, physicians should contact the CDC Emergency Operations Center (EOC) at 1-800-232-4636 (CDC-INFO).
5.10 Prevention of Transmission of Live Vaccinia Virus
The most important measure to prevent inadvertent auto-inoculation and contact transmission from ACAM2000 vaccination is thorough hand washing after changing the bandage or after any other contact with the vaccination site.
Individuals susceptible to adverse effects of vaccinia virus, i.e., those with cardiac disease, eye disease, immunodeficiency states, including HIV infection, eczema, pregnant women and infants, should be identified and measures should be taken to avoid contact between those individuals and persons with active vaccination lesions.
Recently vaccinated healthcare workers should avoid contact with patients, particularly those with immunodeficiencies, until the scab has separated from the skin at the vaccination site. However, if contact with patients is unavoidable, vaccinated healthcare workers should ensure the vaccination site is well covered and follow good hand-washing technique. In this setting, the loose gauze held in place with first aid tape may be covered with a semipermeable (semi-occlusive) dressing as an additional barrier. Semipermeable polyurethane dressings are effective barriers to shedding of vaccinia. However, exudate may accumulate beneath the dressing, and care must be taken to prevent viral spread when the dressing is changed. In addition, accumulation of fluid beneath the dressing may increase skin maceration at the vaccination site. Accumulation of exudate may be decreased by first covering the vaccination with dry gauze, then applying the dressing over the gauze.
The gauze and dressing should be changed every 1 to 3 days [see Patient Counseling Information (17)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- Encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia (vaccinia necrosum), generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson syndrome) and eczema vaccinatum. Severe disability, permanent neurological sequelae, and/or death may occur. Death of unvaccinated individuals who have contact with vaccinated individuals. [See Warnings and Precautions (5.1)].
- •
- Myocarditis and/or pericarditis, ischemic heart disease and non-ischemic, dilated cardiomyopathy [see Warnings and Precautions (5.1)].
- •
- Ocular complications [see Warnings and Precautions (5.3)].
6.1 Clinical Trials Experience
The following information regarding the safety of ACAM2000 was derived from three sources: 1) ACAM2000 clinical trial experience (Phase 1, 2 and 3 clinical trials), 2) data compiled during the era of routine smallpox vaccination using other New York City Board of Health (NYCBH) vaccinia vaccines and 3) adverse event data obtained during military and civilian smallpox vaccination programs (2002-2005) that used Dryvax, a formerly licensed live vaccinia virus smallpox vaccine.
- •
- General Disorders and Administration Site Conditions: In the ACAM2000 clinical studies 97% and 92% of vaccinia-naïve and vaccinia-experienced subjects, respectively, experienced one or more adverse event. Common events included injection site reactions (erythema, pruritus, pain and swelling) and constitutional symptoms (fatigue, malaise, feeling hot, rigors and exercise tolerance decreased). Across all ACAM2000 studies 10% of vaccinia-naïve and 3% of vaccinia-experienced subjects experienced at least one severe adverse event (defined as interfering with normal daily activities).
- •
- Nervous System Disorders: Overall, 50% and 34% of vaccinia-naïve subjects and vaccinia-experienced subjects, respectively, reported headaches in ACAM2000 studies. There have been reports of headache following smallpox vaccination which required hospitalization. Although <1% of the subjects in the ACAM2000 program experienced severe headaches, none required hospitalization.
Neurological adverse events assessed among the 2002 - 2005 military (n=590,400) and DHHS (n=64,600) programs temporally associated with smallpox vaccination included headache (95 cases), non-serious limb paresthesias (17 cases) or pain (13 cases) and dizziness or vertigo (13 cases). Serious neurologic adverse events included 13 cases of suspected meningitis, 3 cases of suspected encephalitis or myelitis, 11 cases of Bell palsy, 9 seizures (including 1 death), and 3 cases of Guillain-Barre syndrome. Among these 39 events, 27 (69%) occurred in primary vaccinees and all but 2 occurred within 12 days of vaccination. There have also been cases of photophobia following smallpox vaccination, some of which required hospitalization. - •
- Musculoskeletal and Connective Tissue Disorders: Across all ACAM2000 studies, severe, vaccine-related myalgia was seen in 1% of vaccinia-naïve subjects and <1% of vaccinia-experienced subjects. Other adverse events included back pain, arthralgia and pain in extremity and none occurred with a frequency of more than 2% in either the vaccinia-naïve or vaccinia-experienced populations.
- •
- Blood and Lymphatic System Disorders: The only adverse event occurring at ≥5% in the ACAM2000 studies were lymph node pain and lymphadenopathy. The incidence of severe lymph node pain and lymphadenopathy was <1%.
- •
- Gastrointestinal (GI) Disorders: Commonly reported GI disorders among subjects who received ACAM2000 included nausea and diarrhea (14%), constipation (6%), and vomiting (4%). Severe abdominal pain, nausea, vomiting, constipation, diarrhea and toothache accounted for all the severe adverse events reported and occurred in <1% of subjects.
- •
- Skin and Subcutaneous Tissue Disorders: Erythema and rash were noted in 18% and 8% of subjects respectively. In ACAM2000 subjects 1% of vaccinia-naïve and <1% of vaccinia-experienced subjects experienced at least one severe adverse event. With the exception of one case of contact dermatitis and one case of urticaria, erythema and rash accounted for all severe events.
Generalized rashes (erythematous, papulovesicular, urticarial, folliculitis, nonspecific) are not uncommon following smallpox vaccination and are presumed to be hypersensitivity reactions occurring among persons without underlying illnesses. These rashes are generally self-limited and require little or no therapy, except among patients whose conditions appear to be toxic or who have serious underlying illnesses.
Inadvertent inoculation at other body sites is the most frequent complication of vaccinia vaccination, usually resulting from autoinoculation of the vaccine virus transferred from the site of vaccination. The most common sites involved are the face, nose, mouth, lips, genitalia and anus. Accidental infection of the eye (ocular vaccinia) may result in ocular complications including, but not limited to, keratitis and corneal scarring that may lead to blindness.
Major cutaneous reactions at the site of inoculation, characterized by large area of erythema and induration and streaking inflammation of draining lymphatics may resemble cellulitis. Benign and malignant lesions have been reported to occur at the smallpox vaccination site.
Two randomized, controlled, multi-center Phase 3 trials enrolled 2244 subjects that received ACAM2000 and 737 that received a comparison formerly licensed live vaccinia virus vaccine, Dryvax. Study 1 was conducted in male (66% and 63% for ACAM2000 and Dryvax, respectively) and female (34% and 37% for ACAM2000 and Dryvax, respectively) subjects who previously had not been vaccinated with smallpox vaccine (i.e., vaccinia-naïve subjects).
The majority of subjects were Caucasian (76% and 71% for ACAM2000 and Dryvax, respectively) and the mean age was 23 in both groups with an age range from 18-30 years. Study 2 was conducted in male (50% and 48% for ACAM2000 and Dryvax, respectively) and female (50% and 52% for ACAM2000 and Dryvax, respectively) subjects who had been vaccinated with smallpox vaccine >10 years previously (i.e., vaccinia-experienced subjects). The majority of subjects were Caucasian (78% for both groups) and the mean age was 49 years in both groups with an age range of 31 to 84 years.
Common Adverse Reactions Reported in ACAM2000 Clinical Program
Adverse reactions reported by ≥5% of subjects in either the ACAM2000 or the comparison vaccine group during Phase 3 studies are presented by type of adverse reactions, by baseline vaccination status (vaccinia-naïve versus vaccinia-experienced) and by vaccine group. Severe vaccine-related adverse reactions, defined as interfering with normal daily activities, in vaccinia-naïve subjects were reported by 10% of subjects in the ACAM2000 group and 13% in the comparison group. In the vaccinia-experienced subjects, the incidence of severe vaccine-related adverse reactions was 4% for the ACAM2000 groups and 6% for the comparison group.
| Vaccine | ACAM2000
N=873 n (%) | Dryvax
N=289 n (%) | ACAM2000
N=1371 n (%) | Dryvax
N=448 n (%) |
|---|---|---|---|---|
| Prior Vaccination Status | Vaccinia-Naïve Subjects | Vaccinia-Naïve Subjects | Vaccinia-Experienced Subjects | Vaccinia-Experienced Subjects |
| Note: With the exception of lymphadenopathy, all reactions were listed on a checklist included in subject diaries; therefore, they should be considered solicited. Lymphadenopathy was identified upon patient examination and recorded during a structured interview. In addition to reactions listed above, the following were also included as part of the checklist: chest pain and heart palpitations, but these reactions did not occur in ≥5% of subjects. | ||||
|
At least 1 adverse reaction |
864 (99) |
288 (100) |
1325 (97) |
443 (99) |
|
Blood and lymphatic system disorders |
515 (59) |
204 (71) |
302 (22) |
133 (30) |
|
Lymph node pain |
494 (57) |
199 (69) |
261 (19) |
119 (27) |
|
Lymphadenopathy |
72 (8) |
35 (12) |
78 (6) |
29 (6) |
|
Gastrointestinal disorders |
273 (31) |
91 (31) |
314 (23) |
137 (31) |
|
Nausea |
170 (19) |
65 (22) |
142 (10) |
63 (14) |
|
Diarrhea |
144 (16) |
34 (12) |
158 (12) |
77 (17) |
|
Constipation |
49 (6) |
9 (3) |
88 (6) |
31 (7) |
|
Vomiting |
42 (5) |
10 (3) |
40 (3) |
18 (4) |
|
General disorders and administration site conditions |
850 (97) |
288 (100) |
1280 (93) |
434 (97) |
|
Injection site pruritus |
804 (92) |
277 (96) |
1130 (82) |
416 (93) |
|
Injection site erythema |
649 (74) |
229 (79) |
841 (61) |
324 (72) |
|
Injection site pain |
582 (67) |
208 (72) |
505 (37) |
209 (47) |
|
Fatigue |
423 (48) |
161 (56) |
468 (34) |
184 (41) |
|
Injection site swelling |
422 (48) |
165 (57) |
384 (28) |
188 (42) |
|
Malaise |
327 (37) |
122 (42) |
381 (28) |
147 (33) |
|
Feeling hot |
276 (32) |
97 (34) |
271 (20) |
114 (25) |
|
Rigors |
185 (21) |
66 (23) |
171 (12) |
76 (17) |
|
Exercise tolerance decreased |
98 (11) |
35 (12) |
105 (8) |
50 (11) |
|
Musculoskeletal and connective tissue disorders |
418 (48) |
153 (53) |
418 (30) |
160 (36) |
|
Myalgia |
404 (46) |
147 (51) |
374 (27) |
148 (33) |
|
Nervous system disorders |
444 (51) |
151 (52) |
453 (33) |
174 (39) |
|
Headache |
433 (50) |
150 (52) |
437 (32) |
166 (37) |
|
Respiratory, thoracic, and mediastinal disorders |
134 (15) |
40 (14) |
127 (9) |
42 (9) |
|
Dyspnea |
39 (4) |
16 (6) |
41 (3) |
18 (4) |
|
Skin and subcutaneous tissue disorders |
288 (33) |
103 (36) |
425 (31) |
139 (31) |
|
Erythema |
190 (22) |
69 (24) |
329 (24) |
107 (24) |
|
Rash |
94 (11) |
30 (10) |
80 (6) |
29 (6) |
6.2 Postmarketing Experience
The following adverse reactions have been identified in the postmarketing data for ACAM2000. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
- •
- Cardiac Disorders: Myopericarditis
- •
- Immune System Disorders: Hypersensitivity, Satellite lesions
- •
- Infections and Infestations: Secondary transmission, Generalised Vaccinia, Eczema Vaccinatum, Encephalomyelitis, Superinfection
- •
- Nervous System Disorders: Encephalopathy
- •
- Skin and subcutaneous tissue disorders: Rash papular, Erythema multiforme, Stevens-Johnson Syndrome
7. Drug Interactions
Interference with Laboratory Tests
During the first 6 weeks after vaccination, ACAM2000 may induce false-positive tests for syphilis. Positive rapid plasma reagin (RPR) tests results should be confirmed using a more specific test, such as the fluorescent treponemal antibody absorption (FTA-ABS) assay.
ACAM2000 may induce temporary false-negative results for the tuberculin skin test (purified protein derivative [PPD]) and possibly, blood tests for tuberculosis. Tuberculin testing should be delayed, if possible, for 6 weeks following smallpox vaccination.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ACAM2000 during pregnancy. Healthcare providers, state health departments, and other public health staff should report to the National Smallpox Vaccine in Pregnancy Registry (NSVIPR) all pregnant women who, from 42 days prior to conception onward, received ACAM2000 or had close contact with a person who received ACAM2000 within the previous 28 days. Civilian women should contact their healthcare provider or state health department for help enrolling in the registry. All civilian and military cases should be reported to the DoD, telephone 619-553-9255, Defense Switched Network (DSN) 553-9255, fax 619-767-4806 or e-mail usn.nhrc-VaccineRegistry@health.mil.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
ACAM2000 has not been studied in pregnant women. Based on data from historical use of other live vaccinia virus vaccines, ACAM2000 can cause fetal harm when administered to a pregnant woman (see below in Fetal/Neonatal Adverse Reactions).
Clinical Considerations
ACAM2000 is not recommended for administration to pregnant women in non-emergency situations. If ACAM2000 is used during pregnancy or within 6 weeks before becoming pregnant, or if the vaccinee lives in the same household with or has close contact with a pregnant woman, the pregnant individual should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.7) and Patient Counselling Information (17)].
Disease-Associated Maternal and/or Embryo/Fetal Risk
Disease caused by smallpox (variola virus) can cause severe illness during pregnancy. Adverse pregnancy outcomes including spontaneous abortion and stillbirth have occurred after smallpox and mpox maternal infections.
Fetal/Neonatal Adverse Reactions
Congenital infection, principally occurring during the first trimester, was observed after vaccination with live vaccinia smallpox vaccines during the era of routine smallpox vaccination. Generalized vaccinia of the fetus, early delivery of a stillborn infant, and perinatal death have been reported from the historical experience with other live vaccinia smallpox vaccines.
8.2 Lactation
Risk Summary
ACAM2000 has not been studied in lactating women.
It is not known whether ACAM2000 is excreted in human milk. No human or animal data are available to assess the effects of ACAM2000 on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ACAM2000 and any potential adverse effects on the breastfed child from ACAM2000 or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
Clinical Considerations
Persons vaccinated with ACAM2000 and who have close contact with infants (e.g., breastfeeding) must take precautions to avoid inadvertent transmission of live vaccinia virus to infants, which may result in serious complications [see Warnings and Precautions (5.6)].
8.3 Females and Males of Reproductive Potential
Contraception
An individual vaccinated with ACAM2000 should be counseled to avoid becoming pregnant (or getting their partner pregnant) for 6 weeks after vaccination.
8.4 Pediatric Use
ACAM2000 has not been studied in the pediatric population. The safety and effectiveness of ACAM2000 in all pediatric age groups is based on the safety and effectiveness of ACAM2000 in adults, historical data on safety and effectiveness of live vaccinia virus smallpox vaccines in pediatric populations, and efficacy of ACAM2000 in protecting non-human primates from lethal challenge with mpox virus. Before the eradication of smallpox disease, live vaccinia virus smallpox vaccines were administered routinely in all pediatric age groups, including neonates and infants, and were effective in preventing smallpox disease. During that time, live vaccinia virus was occasionally associated with serious complications in children, the highest risk being in infants younger than 12 months of age.
11. ACAM2000 Description
ACAM2000 (Smallpox and Mpox (Vaccinia) Vaccine, Live), for scarification suspension for percutaneous use, is a live vaccinia virus vaccine. The virus is derived from plaque purification cloning from Dryvax (Wyeth Laboratories, Marietta, PA, calf lymph vaccine, New York City Board of Health Strain). The vaccine virus is grown in African Green Monkey kidney (Vero) cells and tested to be free of adventitious agents.
ACAM2000 is provided as a Lyophilized Antigen Component, Live of purified live virus containing the following excipients: 6-8 mM HEPES (pH 6.5-7.5), 2% human serum albumin USP, 0.5 – 0.7% sodium chloride USP, 5% mannitol USP, and trace amounts of neomycin and polymyxin B.
The Diluent Component for ACAM2000 contains 50% (v/v) Glycerin USP, 0.25% (v/v) Phenol USP in Water for Injection USP, supplied in clear glass vials containing 0.6 mL of diluent.
After reconstitution, each vial of ACAM2000 contains approximately 100 doses. A single dose is approximately 0.0025 mL.
The concentration of vaccinia virus is 1.0 - 5.0 x 108 plaque-forming units (PFU)/mL or 2.5-12.5 x 105 PFU/dose determined by plaque assay in Vero cells. ACAM2000 is administered by the percutaneous route (scarification) using 15 jabs of a stainless steel bifurcated needle that has been dipped into the vaccine.
12. ACAM2000 - Clinical Pharmacology
12.1 Mechanism of Action
Vaccinia virus is a member of the same taxonomic group (the Orthopoxvirus genus) as variola (which causes smallpox) and mpox viruses. Immunity induced by vaccinia virus cross-protects against variola and mpox viruses. Vaccinia virus causes a localized virus infection of the epidermis at the site of inoculation, surrounding dermal and subcutaneous tissues, and draining lymph nodes. Virus may be transiently present in blood and infects reticuloendothelial and other tissues. Langerhans cells in the epidermis are specific targets for the early stage of virus replication. The formation of a pustule (‘pock’ or ‘take’) at the site of inoculation provides evidence of protective immunity. The virus replicates within cells and viral antigens are presented to the immune system. Neutralizing antibodies and B and T cells provide long-term memory. The level of neutralizing antibody that protects against smallpox or mpox is unknown but >95% of persons undergoing primary vaccination develop neutralizing or hemagglutination inhibiting antibodies to vaccinia.
12.2 Pharmacodynamics
Cutaneous Response
The cutaneous responses following ACAM2000 vaccination are dependent on the immune status of the individual, potency of the vaccine, and vaccination technique. Two types of responses have been defined by the WHO Expert Committee on Smallpox and described by the CDC - Advisory Committee on Immunization Practices (ACIP). The responses include: a) major cutaneous reaction, which indicates that virus replication has taken place and vaccination was successful; or b) equivocal reaction. Equivocal reactions may be a consequence of pre-existing immunity adequate to suppress viral multiplication, vaccination technique failure, or use of inactive vaccine or vaccine that has lost potency.
Successful vaccination in persons who are vaccinia vaccine-naïve, termed primary vaccination, is represented by a major cutaneous reaction, defined as a vesicular or pustular lesion or an area of definite palpable induration or congestion surrounding a central lesion that might be a crust or an ulcer.
Persons who have been previously vaccinated (vaccinia-experienced) and are revaccinated may manifest a reduced cutaneous response compared to vaccinia vaccine naïve persons, but still exhibit an immune response to the vaccine. [see Dosage and Administration (2.7)].
Neutralizing Antibody and Cellular Immune Responses
Neutralizing antibodies are known to mediate protection against smallpox. The level of neutralizing antibody that is required to protect against smallpox and mpox has not been clearly established. Some studies indicate that persons with antibody titers > 1:32 are protected against smallpox. Neutralizing antibodies against vaccinia develop in >95% of individuals following primary vaccination, rise rapidly (by day 15-20 after vaccination) and may be boosted on revaccination. Antibody titers are highly variable. Titers may remain high for longer periods following two or more vaccinations than after a primary vaccination. The level of the neutralizing antibody response following primary vaccination is generally in proportion to the intensity of the cutaneous reaction. Cellular immune responses are also elicited by vaccination and may contribute to protection and immunological memory.
Virus Shedding
Virus is shed from the vaccination site during the period starting with the development of a papule (day 2-5); shedding ceases when the scab separates and the lesion is re-epithelialized, typically 3-6 weeks after vaccination. Steps should be taken in clinical use to reduce the risk of accidental infection of other sites in the vaccinated patient or of contact spread to other individuals [see Dosage and Administration (2.6) and Warnings and Precautions (5.10)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
ACAM2000 has not been evaluated for carcinogenic or mutagenic potential, or for impairment of male or female fertility in animals.
13.2 Animal Toxicology and/or Pharmacology
The efficacy of ACAM2000 to protect Cynomolgus macaques (Macaca fascicularis) against mpox virus challenge was evaluated. Animals were administered placebo (ACAM2000 Diluent Component), ACAM2000 or Dryvax percutaneously (approximately 0.0025 mL each via scarification) on Day 0. On Day 61, all animals were challenged with an intravenous injection of mpox virus (3.8x107 PFU). 100% of animals vaccinated with ACAM2000 or Dryvax survived compared to 0% of animals that received placebo.
14. Clinical Studies
The effectiveness of ACAM2000 for the prevention of mpox is based on its effectiveness for the prevention of smallpox and efficacy in animal challenge studies [see Nonclinical Toxicology (13.2)].
The effectiveness of ACAM2000 for the prevention of smallpox was assessed by comparing the immunologic response of ACAM2000 to a previously U.S.-licensed live vaccinia virus smallpox vaccine, Dryvax, in two randomized, multi-center active-controlled clinical trials; one study in subjects who previously had not been vaccinated with smallpox vaccine (i.e., vaccinia-naïve subjects) and one study in subjects who had been vaccinated with smallpox vaccine >10 years previously (i.e., vaccinia-experienced subjects). In both trials, the co-primary efficacy endpoints were the proportion of subjects with a successful vaccination/revaccination and the geometric mean neutralizing antibody titer (GMT) on Day 30. Successful primary vaccination was defined as a major cutaneous reaction on Day 7 or 10 (Days 6 to 11, with allowable visit window). Successful revaccination was defined as development of any cutaneous lesion on Day 7 (± 1 day) of a measurable size. Successful revaccination was determined by a panel of experts who reviewed digital photographs of the cutaneous lesions.
The statistical method used to compare the proportion of subjects who were successfully vaccinated in the two vaccine groups was a test of noninferiority of ACAM2000 to Dryvax intended to rule out a greater than 5% margin of superiority of Dryvax for successful primary vaccination (Study 1) and a 10% margin of superiority of the comparator for successful revaccination (Study 2). Noninferiority was to be declared if the lower bound of the 1-sided 97.5% confidence interval (CI) for the percent difference between ACAM2000 and Dryvax exceeded -5% in vaccinia-naïve subjects and 10% in vaccinia-experienced subjects.
Analysis of the GMT was performed using a test of noninferiority of neutralizing antibody titer between ACAM2000 and Dryvax, intended to ensure that the ratio of the GMTs of ACAM2000: Dryvax was at least 0.5 (equivalent to the difference of the log10 (GMT) being at least -0.301).
In Study 1, a total of 1037 male and female vaccinia-naïve subjects, aged 18 to 30 years inclusive, primarily Caucasian (76%) were randomized in a 3:1 ratio to receive ACAM2000 (780 subjects) or comparator Dryvax (257 subjects). The ACAM2000 vaccinated subjects were further stratified to receive one of three lots (Lots A, B and C) at a 1:1:1 ratio (258, 264, and 258 subjects, respectively). All subjects were to be evaluated for their cutaneous response and a random subset was selected for evaluation of neutralizing antibody response.
In Study 2, a total of 1647 male and female vaccinia-experienced subjects, aged 31 to 84 years inclusive, primarily Caucasian (81%) were randomized in a 3:1 ratio to receive ACAM2000 (1242 subjects) or Dryvax (405 subjects). The ACAM2000 vaccinated subjects were further stratified to receive one of three lots (Lots A, B and C) at a 1:1:1 ratio (411, 417, and 414 subjects, respectively). All subjects were evaluated for their cutaneous response and a random subset was to be selected for evaluation of neutralizing antibody response.
Table 5 and Table 6 present the results of the primary efficacy analyses for both studies.
| Clinical Study | Study 1 ACAM2000 | Study 1 Dryvax | Study 2 ACAM2000 | Study 2 Dryvax |
|---|---|---|---|---|
| a Subjects who received study vaccine and were evaluated for a local cutaneous reaction within the protocol designated timeframe (assessment of local cutaneous reaction between Days 6 and 11 in Study 1 and Days 6 to 8 in Study 2) were included in the efficacy evaluable (EE) population. | ||||
| b Since the critical value for the evaluation was declared to be -5%, ACAM2000 is considered to be non-inferior to Dryvax for this parameter. | ||||
| c Since the critical value for the evaluation was declared to be -10%, ACAM2000 is not considered to be non-inferior to Dryvax for this parameter. | ||||
|
Size of Efficacy Evaluable (EE) Population(a) |
776 |
257 |
1189 |
388 |
|
Number of Vaccination Successes (%) |
747 (96) |
255 (99) |
998 (84) |
381 (98) |
|
97.5% 1-sided CI by normal approx. on percent difference between ACAM2000 and Dryvax |
-4.67% (b) |
-- |
-17% (c) |
-- |
|
Non-Inferiority to Dryvax |
Yes |
-- |
No |
-- |
| Clinical Study | Study 1 ACAM2000 | Study 1 Dryvax | Study 2 ACAM2000 | Study 2 Dryvax |
|---|---|---|---|---|
| a A randomly selected sample of subjects who received study vaccine and had samples collected for neutralizing antibody response at Baseline and at the designated timepoint post vaccination were included in the antibody evaluable (AnE) population. | ||||
| b GMT – Geometric mean neutralizing antibody titer as measured by Vaccinia 50% plaque reduction neutralization test. | ||||
| c Since the critical value for the evaluation was declared to be -0.301, ACAM2000 is not considered to be non-inferior to Dryvax for this parameter. | ||||
| d Since the critical value for the evaluation was declared to be -0.301, ACAM2000 is considered to be non-inferior to Dryvax for this parameter | ||||
|
Size of Antibody Evaluable Population(a) |
565 |
190 |
734 |
376 |
|
GMT(b) |
166 |
255 |
286 |
445 |
|
Log10 mean |
2.2 |
2.4 |
2.5 |
2.6 |
|
97.5% 1-sided CI by ANOVA on difference between ACAM2000 and Dryvax |
-0.307(c) |
-- |
-0.275(d) |
-- |
|
Meets Non-Inferiority to Dryvax |
No |
-- |
Yes |
-- |
The primary determinant for an effective immune response in those naïve to vaccine is a major cutaneous reaction. An efficacy evaluable (EE) population was used for determination of vaccination success. ACAM2000 was non-inferior to Dryvax in the EE population when eliciting a major cutaneous reaction. The measure of the strength of the generated antibody response in vaccinia-naïve subjects was similar between vaccine groups but did not meet the predefined criterion for non-inferiority. Among subjects who were vaccinia-experienced, development of a major cutaneous response after revaccination with vaccinia-based vaccines may not provide an accurate measure of the strength of the immune response because the preexisting immunity modifies the scope of the cutaneous response. In vaccinia-experienced subjects, ACAM2000 did not meet the non-inferiority criterion for cutaneous response but was non-inferior to Dryvax with regard to the strength of the neutralizing antibody immune response. Therefore, ACAM2000 was non-inferior to Dryvax in the rate of major cutaneous reaction in those naïve to the vaccine only, and non-inferior to Dryvax in the strength of the neutralizing antibody immune response in those previously exposed to vaccinia-based vaccines.
15. References
- 1.
- “Comparison of Reactions for First-Time Vaccinees and Revaccinees,” Centers for Disease Control and Prevention, 5 December 2016. [Online]. Materials developed by United States Center for Disease Control and Prevention (CDC). Reference to specific commercial products, manufacturers, companies, or trademarks does not constitute its endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention. The material is otherwise available on the agency website for no charge. Available: https://www.cdc.gov/smallpox/clinicians/comparison-vaccinees-images.html [Accessed 24 November 2022].
- 2.
- Petersen BW, Harms TJ, Reynolds MG, Harrison LH. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses – Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016; 65(10):257-262.
- 3.
- Lane J, Millar J. Risks of smallpox vaccination complications in the United States. Am J Epidemiol. 1971;93:238-240.
- 4.
- Lane, JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970;122(4):303-309.
- 5.
- Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005 Mar 18;23(17-18):2078-81. doi: 10.1016/j.vaccine.2005.01.012. PMID: 15755574.
16. How is ACAM2000 supplied
16.1 How Supplied
ACAM2000 is supplied as 2 components as follows:
- •
- Lyophilized Antigen Component, Live (labels state: Smallpox (Vaccinia) Vaccine, Live
ACAM2000®)- o
- Vial (NDC 71665-430-01 and NDC 71665-330-01)
- o
- Each carton (NDC 71665-430-02 and NDC 71665-330-02) contains 50 multiple-dose clear glass vials
- o
- Each case (NDC 71665-430-03 and NDC 71665-330-03) contains 8 cartons
- •
- Diluent Component (labels may state: Diluent for Smallpox (Vaccinia) Vaccine, Live,
ACAM2000®)- o
- Vial (NDC 71665-440-01 and NDC 71665-340-01)
- o
- Each carton (NDC 71665-440-02 and NDC 71665-340-02) contains 50 clear glass vials
- o
- Each case (NDC 71665-440-03 and NDC 71665-340-03) contains 8 cartons
Bifurcated needles are supplied separately. Tuberculin syringes and needles (1 mL, 25-gauge x 5/8” needles) for vaccine reconstitution are supplied separately.
The stoppers of the vials containing the Lyophilized Antigen Component, Live and the stoppers of the vials containing the Diluent Component are not made with natural rubber latex.
16.2 Storage and Handling
The Lyophilized Antigen Component, Live is supplied in cartons and vials labeled “Smallpox (Vaccinia) Vaccine, Live, ACAM2000.” The Diluent Component may be supplied in cartons and vials labeled “Diluent for Smallpox (Vaccinia) Vaccine, Live, ACAM2000.” Storage and Handling conditions are described below.
Lyophilized Antigen Component, Live
Lyophilized Antigen Component, Live is shipped frozen. During shipment, the Lyophilized Antigen Component, Live should be maintained at a temperature of -10°C (14°F) or colder.
Store in a freezer with an average temperature of -25°C to -15°C (-13°F to 5°F).
The Lyophilized Antigen Component, Live may be stored at refrigerated temperatures of 2°C to 8°C (36°F to 46°F) for up to 18 months or until expiry whichever comes first. Calculate and record the 18-month expiry date when Lyophilized Antigen Component, Live is moved from freezer to refrigerator.
Diluent Component
The Diluent Component is supplied in separate cartons and is shipped at 2°C to 30°C (36°F to 86°F).
Store at 15°C to 30°C (59°F to 86°F).
Reconstituted ACAM2000
After reconstitution, store in a refrigerator at 2°C to 8°C (36°F to 46°F) for up to 30 days. ACAM2000 may be kept at 20°C to 25°C (68°F to 77°F) for up to 8 hours. Discard unused reconstituted ACAM2000 as a biohazardous material [see Dosage and Administration (2.5)].
ACAM2000 contains live vaccinia virus that is transmissible and should be handled as an infectious agent once vials are open [see Dosage and Administration (2.3 and 2.5)].
17. Patient Counseling Information
Instruct the vaccine recipient or caregiver to read the FDA-approved Medication Guide for ACAM2000. Provide pregnancy registry information to individuals vaccinated with ACAM2000 who are or may become pregnant [see Use in Specific Populations (8.1)].
Serious Complications of Vaccination
Vaccine recipients or caregivers must be informed of the major serious adverse reactions associated with vaccination, including myocarditis and/or pericarditis, progressive vaccinia in immunocompromised persons, eczema vaccinatum in persons with skin disorders, auto- and accidental inoculation, generalized vaccinia, urticaria, erythema multiforme major (including Stevens-Johnson syndrome) and fetal vaccinia in pregnant women.
Protecting Contacts at Highest Risk for Adverse Events
Vaccine recipients or caregivers must be informed that they should avoid contact with individuals at high risk of serious adverse effects of vaccinia virus, for instance, those with past or present eczema, immunodeficiency states including HIV infection, pregnancy, or infants less than 12 months of age.
Self-inoculation and Spread to Close Contacts
Vaccine recipients or caregivers must be advised that virus is shed from the cutaneous lesion at the site of inoculation from approximately Day 2 post-vaccination until the scab separates and the lesion is re-epithelialized typically 3 to 6 weeks after primary vaccination. Vaccinia virus may be transmitted by direct physical contact. Accidental infection of skin at sites other than the site of intentional vaccination (self-inoculation) may occur by trauma or scratching. Contact spread may also result in accidental inoculation of household members or other close contacts (including sexual contacts). The result of accidental infection is a pock lesion(s) at an unwanted site(s) in the vaccinee or contact and resembles the vaccination site. Self-inoculation occurs most often on the face, eyelid, nose, anus and mouth, but lesions at any site of traumatic inoculation can occur. Self-inoculation of the eye may result in ocular vaccinia, a potentially serious complication.
Care of the Vaccination Site and Potentially Contaminated Materials
Vaccine recipients or caregivers must be given the following instructions:
- •
- The vaccination site must be completely covered with gauze secured loosely with first aid adhesive tape. If the vaccine recipient is directly involved in patient care, the gauze may be covered with a semipermeable dressing that allows passage of air but not fluids. Keep the site covered until the scab falls off on its own.
- •
- The vaccination site must be kept dry. Normal bathing may continue, but cover the vaccination site with waterproof bandage when bathing. Do not scrub the site. Cover the vaccination site with loose gauze held in place with first aid adhesive tape after bathing.
- •
- Don’t scratch the vaccination site. Don’t scratch or pick at the scab.
- •
- Do not touch the lesion or soiled gauze, semipermeable dressing, or bandages and then touch other parts of the body such as the eyes, anal and genital areas where the virus can spread.
- •
- After changing the gauze, semipermeable dressing, or bandages, or touching the site, wash hands thoroughly with soap and water or >60% alcohol-based hand-rub solutions.
- •
- To prevent transmission to contacts, physical contact of objects that have come into contact with the lesion (e.g., soiled bandages, clothing, fingers) must be avoided.
- •
- Wash separately clothing, towels, bedding or other items that may have come in direct contact with the vaccination site or drainage from the site, with using hot water with detergent and/or bleach. Wash hands afterwards.
- •
- Soiled and contaminated gauze, semipermeable dressings, and bandages must be placed in plastic bags for disposal.
- •
- The vaccinee must wear a shirt with sleeves that covers the vaccination site as an extra precaution to prevent spread of the vaccinia virus. This is especially important in case of close physical contact with others.
- •
- The vaccinee must change the gauze and semipermeable dressing every 1 to 3 days. This will keep skin at the vaccination site intact and minimize softening.
- •
- Do not put salves or ointments on the vaccination site.
- •
- When the scab falls off, throw it away in a sealed plastic bag and wash hands afterwards.
Manufactured by
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD USA 20879
License No. 2089
Any and all Emergent BioSolutions Inc. brand, product, service and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All rights reserved.
LBL040018-02
Health Care Provider Letter
02/2025
IMPORTANT PRESCRIBING INFORMATION
|
Subject: ACAM2000® [Smallpox and Mpox (Vaccinia) Vaccine, Live] supplied in vials, cartons and cases with previously approved labels |
Dear Health Care Provider:
The purpose of this letter is to notify you that you have received ACAM2000 supplied in vials, cartons, and cases with previously approved labels.
The previously approved labels do not include:
- •
- The revised nonproprietary name for ACAM2000: Smallpox and Mpox (Vaccinia) Vaccine, Live
- •
- The revised names for the two components of ACAM2000 (i.e., Lyophilized Antigen Component, Live and Diluent Component).
The previously approved labels include the previous nonproprietary name for ACAM2000: Smallpox (Vaccinia) Vaccine, Live and refer to the Diluent Component as “Diluent for Smallpox (Vaccinia) Vaccine, Live, ACAM2000®.”
The components of ACAM2000 have not changed despite the revised names. The revised names for the vaccine components are used in the US Prescribing Information (USPI) including in the Dosage and Administration and the How Supplied/Storage and Handling sections. Complete reconstitution instructions and storage and handling are described in the USPI.
You will continue to receive ACAM2000 components packaged with the previously approved vial, carton, and case labels until this supply is depleted or expired.
The ACAM2000 nonproprietary name was revised when the indication for ACAM2000 vaccine was expanded on August 29, 2024, to include the prevention of mpox disease in individuals determined to be at high risk for mpox infection. This required revisions to the USPI and Medication Guide to include the new nonproprietary name and indication. Updates were also made to the Dosage and Administration and How Supplied/Storage and Handling sections of the USPI. Changes were made to the product packaging labelling (vial, carton and case labels) to make them consistent with the USPI.
Indications and Usage
ACAM2000 is a vaccine indicated for active immunization for the prevention of smallpox and mpox disease in individuals determined to be at high risk for smallpox or mpox infection.
Reporting Adverse Events
Report adverse events following use of ACAM2000 to Emergent BioSolutions at 1-877-246-8472 and medicalinformation@ebsi.com or to VAERS at 800-822-7967 and https://vaers.hhs.gov.
You may contact our medical information department if you have any questions about the information contained in this letter or the safe and effective use of ACAM2000 at 1-877-246-8472 or medicalinformation@ebsi.com
This letter is not intended as a complete description of the benefits and risks related to the use of ACAM2000; please refer to the enclosed full prescribing information and Medication Guide.
Sincerely,
Company Representative
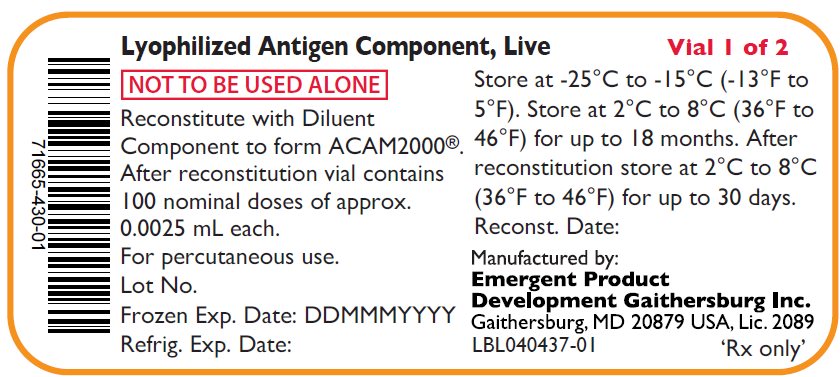
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Antigen Vial Labels
Lyophylized Antigen Component, Live
NOT TO BE USED ALONE
Reconstitute with Diluent
Component to form ACAM2000®.
After reconstitution vial contains
100 nominal doses of approx..
0.0025 mL each.
For percutaneous use.
Lot No.
Frozen Exp. Date: DDMMMYYYY
Refrig. Exp. Date:
Vial 1 of 2
Store at -25°C to -15°C (-13°F to
5°F). Store at 2°C to 8°C (36°F to
46°F) for up to 18 months. After
reconstitution store at 2°C to 8°C
(36°F to 46°F) for up to 30 days.
Reconst. Date:
Manufactured by:
Emergent Product
Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
LBL040437-01
‘Rx only’
STRATEGIC NATIONAL STOCKPILE USE ONLY
Smallpox (Vaccinia) Vaccine,
Live ACAM2000®
'Rx Only'. For percutaneous
inoculation with Bifurcated Needle
only. Do not administer by injection.
Contains trace amounts of antibiotics.
Lot No.
Mfg. Date:
Recon Date:
Store at 2-8°C (36-46°F). Use within
30 days of reconstitution.
Fill volume: 0.3 mL/100 doses.
Approximate Dose: 2.5µ L
Potency: 1-5x108 PFU/mL
Manufactured by:
Emergent Product
Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
COMPONENT # LBL040020-01
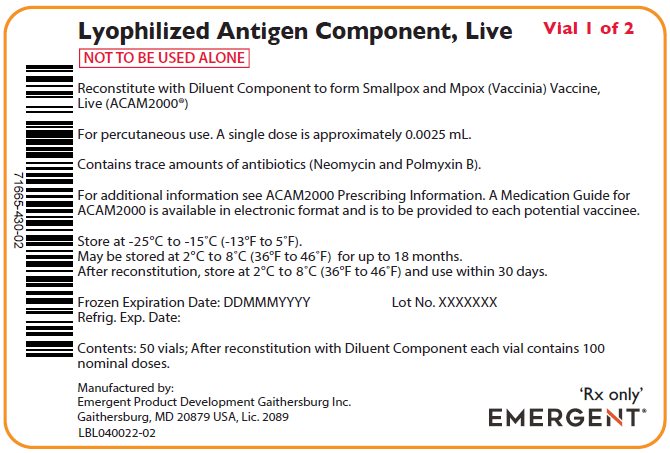
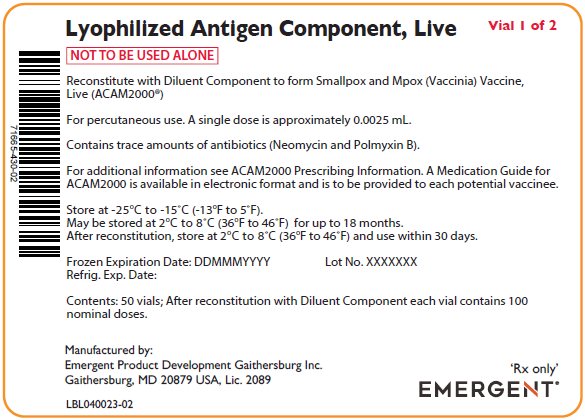
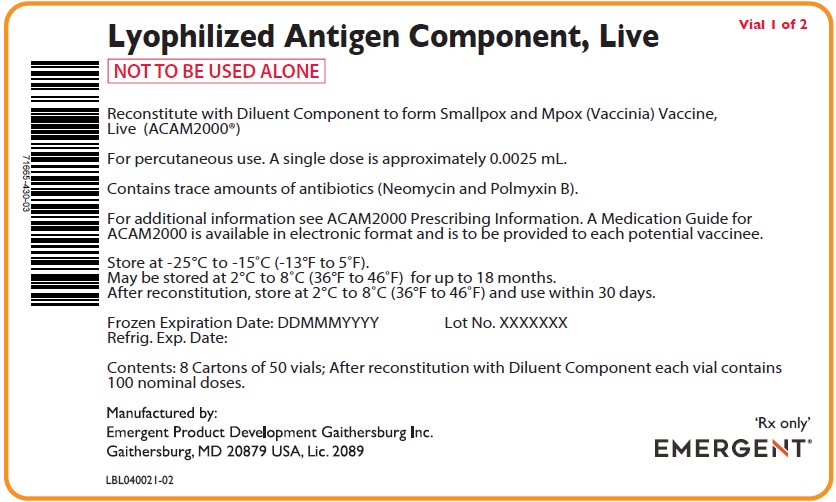
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Antigen Carton Labels
Lyophylized Antigen Component, Live
Vial 1 of 2
NOT TO BE USED ALONE
Reconstitute with Diluent Component to form Smallpox and Mpox (Vaccinia) Vaccine,
Live (ACAM2000®)
For percutaneous use. A single dose is approximately 0.0025 mL.
Contains trace amounts of antibiotics (Neomycin and Polmyxin B).
For additional information see ACAM2000 Prescribing Information. A Medication Guide for
ACAM2000 is available in electronic format and is to be provided to each potential vaccinee.
Store at -25°C to -15°C (-13°F to 5°F).
May be stored at 2°C to 8°C (36°F to 46°F) for up to 18 months.
After reconstitution, store at 2°C to 8°C (36°F to 46°F) and use within 30 days.
Frozen Expiration Date: DDMMMYYYY Lot No. XXXXXXX
Refrig. Exp. Date:
Contents: 50 vials; After reconstitution with Diluent Component each vial contains 100
nominal doses.
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
LBL040022-02
‘Rx only’
EMERGENT®
STRATEGIC NATIONAL STOCKPILE USE ONLY
Smallpox (Vaccinia) Vaccine, Live
ACAM2000®
'Rx Only'. For percutaneous inoculation with Bifurcated Needle only. Do not administer
by injection. Live Vaccinia virus derived from cloning Dryvax® (Wyeth Laboratories,
NYCBH Strain) and grown in Vero cells. For full prescribing and product information, see
ACAM2000® Package Insert.
Contains trace amounts of antibiotics (Neomycin and Polymyxin B).
A Medication Guide for ACAM2000® is available in electronic format, and is to be
provided to each potential vaccinee.
Approximate dose: 2.5 µL
Lot No. XXXXXXX
Potency: 1-5x108 PFU/mL
Expiration Date: DDMMMYYYY
Store at -25 to -15°C (-13 to 5°F) until distributed for use. Upon distribution, product
may be stored at 2-8°C (36-46°F) for up to 18 months. Do not use after expiration date.
Store reconstituted vaccine at 2-8°C (36-46°F). Use within 30 days of reconstitution date.
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
Contents: 50 Vials; 0.3 mL/100 doses/vial
Component # LBL040022-01
emergent
biosolutions®
Lyophylized Antigen Component, Live
Vial 1 of 2
NOT TO BE USED ALONE
Reconstitute with Diluent Component to form Smallpox and Mpox (Vaccinia) Vaccine,
Live (ACAM2000®)
For percutaneous use. A single dose is approximately 0.0025 mL.
Contains trace amounts of antibiotics (Neomycin and Polmyxin B).
For additional information see ACAM2000 Prescribing Information. A Medication Guide for
ACAM2000 is available in electronic format and is to be provided to each potential vaccinee.
Store at -25°C to -15°C (-13°F to 5°F).
May be stored at 2°C to 8°C (36°F to 46°F) for up to 18 months.
After reconstitution, store at 2°C to 8°C (36°F to 46°F) and use within 30 days.
Frozen Expiration Date: DDMMMYYYY Lot No. XXXXXXX
Refrig. Exp. Date:
Contents: 50 vials; After reconstitution with Diluent Component each vial contains 100
nominal doses.
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
LBL040023-02
‘Rx only’
EMERGENT®
STRATEGIC NATIONAL STOCKPILE USE ONLY
Smallpox (Vaccinia) Vaccine, Live
ACAM2000®
'Rx Only'. For percutaneous inoculation with Bifurcated Needle only. Do not administer
by injection. Live Vaccinia virus derived from cloning Dryvax® (Wyeth Laboratories,
NYCBH Strain) and grown in Vero cells. For full prescribing and product information, see
ACAM2000® Package Insert.
Contains trace amounts of antibiotics (Neomycin and Polymyxin B).
A Medication Guide for ACAM2000® is available in electronic format, and is to be
provided to each potential vaccinee.
Approximate dose: 2.5 µL
Lot No. XXXXXXX
Potency: 1-5x108 PFU/mL
Expiration Date: DDMMMYYYY
Store at -25 to -15°C (-13 to 5°F) until distributed for use. Upon distribution, product
may be stored at 2-8°C (36-46°F) for up to 18 months. Do not use after expiration date.
Store reconstituted vaccine at 2-8°C (36-46°F). Use within 30 days of reconstitution date.
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
Contents: 50 Vials; 0.3 mL/100 doses/vial
Component # LBL040023-01
emergent
biosolutions®
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Antigen Case Labels
Lyophylized Antigen Component, Live
Vial 1 of 2
NOT TO BE USED ALONE
Reconstitute with Diluent Component to form Smallpox and Mpox (Vaccinia) Vaccine,
Live (ACAM2000®)
For percutaneous use. A single dose is approximately 0.0025 mL.
Contains trace amounts of antibiotics (Neomycin and Polmyxin B).
For additional information see ACAM2000 Prescribing Information. A Medication Guide for
ACAM2000 is available in electronic format and is to be provided to each potential vaccinee.
Store at -25°C to -15°C (-13°F to 5°F).
May be stored at 2°C to 8°C (36°F to 46°F) for up to 18 months.
After reconstitution, store at 2°C to 8°C (36°F to 46°F) and use within 30 days.
Frozen Expiration Date: DDMMMYYYY Lot No. XXXXXXX
Refrig. Exp. Date:
Contents: 8 Cartons of 50 vials; After reconstitution with Diluent Component each vial contains
100 nominal doses.
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
LBL040021-02
‘Rx only’
EMERGENT®
STRATEGIC NATIONAL STOCKPILE USE ONLY
Smallpox (Vaccinia) Vaccine, Live
ACAM2000®
'Rx Only'. For percutaneous inoculation with Bifurcated Needle only. Do not administer by injection.
Live Vaccinia virus derived from cloning Dryvax® (Wyeth Laboratories, NYCBH Strain) and grown in
Vero cells. For full prescribing and product information, see ACAM2000® Package Insert.
Contains trace amounts of antibiotics (Neomycin and Polymyxin B).
A Medication Guide for ACAM2000® is available in electronic format, and is to be
provided to each potential vaccinee.
Approximate dose: 2.5 µL
Lot No. XXXXXXX
Potency: 1-5x108 PFU/mL
Expiration Date: DDMMMYYYY
Store at -25 to -15°C (-13 to 5°F) until distributed for use. Upon distribution, product may
be stored at 2-8°C (36-46°F) for up to 18 months. Do not use after expiration date. Store
reconstituted vaccine at 2-8°C (36-46°F). Use within 30 days of reconstitution date.
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA, Lic. 2089
Contents: 8 Boxes of 50 Vials; 0.3 mL/100 doses/vial
Component # LBL040021-01
emergent
biosolutions®
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Diluent Vial Labels
Diluent Component
NOT TO BE USED ALONE
Add 0.3 mL to one of
Lyophilized Antigen
Component, Live to form
ACAM2000®
Lot No. / Exp. Date:
Vial 2 of 2
Store at 15°C to 30°C
(59°F to 86°F)
50% (v/v) Glycerin USP
0.25% (v/v) Phenol USP
Manufactured by:
Emergent Product Development
Gaithersburg Inc.
Gaithersburg MD 20879 USA
LBL040438-01
Diluent for Smallpox
(Vaccinia) Vaccine, Live,
ACAM2000®
50% (v/v) Glycerin USP
0.25% (v/v) Phenol USP
in Water for Injection USP
Lot No. / Exp. Date:
Store at 15-30°C (59-86°F)
For vaccine reconstitution –
do not administer by injection
Fill Volume: 0.6mL – Transfer
0.3mL to vaccine vial
Manufactured by:
Emergent Product Development
Gaithersburg Inc.
Gaithersburg MD 20879 USA
50000001 151-H02
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Diluent Carton Labels
Diluent Component
NOT TO BE USED ALONE
Vial 2 of 2
Use to reconstitute Lyophilized Antigen Component, Live to form Smallpox
and Mpox (Vaccinia) Vaccine, Live (ACAM2000®)
50% (v/v) Glycerin USP, 0.25% (v/v) Phenol USP, in Water for Injection USP
Lot No.
Expiration Date:
Store at 15°C to 30°C (59°F to 86°F)
Contents: 50 Vials of Diluent Component
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA
LBL040025-02
EMERGENT®
Diluent for Smallpox (Vaccinia) Vaccine, Live,
ACAM2000®
50% (v/v) Glycerin USP, 0.25% (v/v) Phenol USP, in Water for Injection USP
For vaccine reconstitution. Do not administer by injection.
Lot No.
Expiration Date:
Store at 15-30°C (59-86°F)
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA
Contents: 50 Vials
876389-H02
emergent
biosolutions®
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Diluent Case Labels
Diluent Component
NOT TO BE USED ALONE
Vial 2 of 2
Use to reconstitute Lyophilized Antigen Component, Live to form Smallpox and
Mpox (Vaccinia) Vaccine, Live (ACAM2000®)
50% (v/v) Glycerin USP, 0.25% (v/v) Phenol USP, in Water for Injection USP
Lot No.
Expiration Date:
Store at 15°C to 30°C (59°F to 86°F)
Contents: 8 Cartons of 50 Vials of Diluent Component
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA
LBL040026-02
EMERGENT
Diluent for Smallpox (Vaccinia) Vaccine, Live,
ACAM2000®
50% (v/v) Glycerin USP, 0.25% (v/v) Phenol USP, in Water for Injection USP
For vaccine reconstitution. Do not administer by injection.
Lot No.
Expiration Date:
Store at 15-30°C (59-86°F)
Manufactured by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD 20879 USA
Contents: 8 Boxes of 50 Vials
876390-H02
emergent
biosolutions®
| ACAM2000
smallpox and mpox (vaccinia) vaccine, live injection, powder, lyophilized, for solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| DILUENT FOR ACAM2000
diluent for smallpox and mpox (vaccinia) vaccine, live solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ACAM2000
smallpox and mpox (vaccinia) vaccine, live injection, powder, lyophilized, for solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| DILUENT FOR ACAM2000
diluent for smallpox and mpox (vaccinia) vaccine, live solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Emergent Product Development Gaithersburg Inc. (189488554) |
More about ACAM2000 (smallpox and mpox vaccine)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: viral vaccines