Hemiclor: Package Insert / Prescribing Info
Package insert / product label
Generic name: chlorthalidone
Dosage form: tablet
Drug class: Thiazide diuretics
Medically reviewed by Drugs.com. Last updated on Apr 7, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
HEMICLOR (chlorthalidone) tablets, for oral use
Initial U.S. Approval: 1960
Indications and Usage for Hemiclor
HEMICLOR is a thiazide-like diuretic indicated for the treatment of hypertension in adults, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions (1.1).
Hemiclor Dosage and Administration
Dosage Forms and Strengths
- Tablets: 12.5 mg (3).
Contraindications
Warnings and Precautions
Adverse Reactions/Side Effects
The most frequently expected adverse drug reactions among patients receiving thiazide-like diuretics are electrolyte abnormalities and metabolic disturbances (6).
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Insulin requirements and oral hypoglycemic agent dosages may require adjustments (7).
- Possible increased responsiveness to tubocurarine (7).
- Possible decreased arterial responsiveness to norepinephrine (7).
- Lithium renal clearance is reduced by chlorthalidone, increasing the risk of lithium toxicity (7).
Use In Specific Populations
- Pregnancy: May cause fetal harm (8.1).
- Lactation: Breastfeeding not recommended (8.2).
- Geriatric Use: No overall difference in responses versus younger patients, but care should be taken in dose selection in patients with impaired renal function (8.5).
- Hepatic Impairment: Alterations on fluid and electrolyte balance should be monitored in patients with hepatic impairment (8.6).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2025
Full Prescribing Information
1. Indications and Usage for Hemiclor
1.1 Hypertension
HEMICLOR is indicated for the treatment of hypertension in adults, to lower blood pressure.
Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic blood pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
HEMICLOR may be used alone or in combination with other antihypertensives.
2. Hemiclor Dosage and Administration
2.1 Recommended Dosage
Therapy should be initiated with the lowest possible dose. The recommended adult initial dose of HEMICLOR is 12.5 mg or 25 mg given orally with food once daily. Double the dosage every 2 to 4 weeks as needed based on individual patient response, up to a maximum of 100 mg once daily. Dosage above 100 mg daily usually does not increase effectiveness.
3. Dosage Forms and Strengths
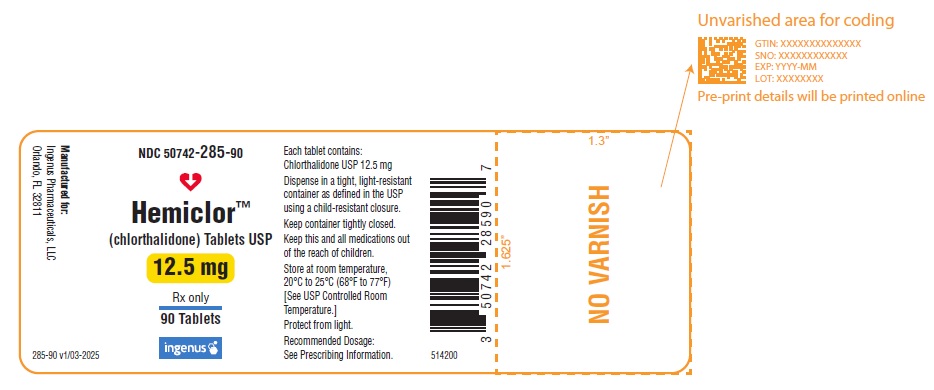
Each HEMICLOR tablet contains 12.5 mg of chlorthalidone. The tablets are white to off-white, modified rectangle shaped tablets debossed with “I” on one side and plain on the other side.
4. Contraindications
Chlorthalidone is contraindicated in patients with anuria, or hypersensitivity to chlorthalidone or other sulfonamide-derived drugs.
5. Warnings and Precautions
5.1 Acute Kidney Injury
Monitor kidney function periodically. Diuretics can cause hypovolemia which may precipitate acute kidney injury. Patients with chronic kidney disease, heart failure, or volume depletion may be at particular risk of developing acute renal failure on Chlorthalidone. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in kidney function while on chlorthalidone.
5.2 Electrolyte Abnormalities
Chlorthalidone can cause hypokalemia, hyponatremia, hypochloremic alkalosis, and hypomagnesemia. Hypomagnesemia can result in hypokalemia which appears difficult to treat despite potassium repletion. Hypokalemia is dose dependent. Monitor and correct serum electrolytes prior to use and monitor periodically.
5.3 Metabolic Disturbances
Chlorthalidone may increase blood sugar levels, affect diabetes control, and cause changes in the need for diabetes medication.
Chlorthalidone may raise serum levels of cholesterol and triglycerides. Monitor blood sugar and lipid levels.
Chlorthalidone may raise the serum uric acid level due to reduced clearance of uric acid and may cause or exacerbate hyperuricemia and precipitate gout in susceptible patients. Increases in serum uric acid are dose related.
Chlorthalidone decreases urinary calcium excretion and may cause elevations of serum calcium. Monitor calcium levels in patients with hypercalcemia receiving Chlorthalidone.
5.4 Systemic Lupus Erythematosus
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported with thiazide diuretics, which are structurally related to chlorthalidone. However, systemic lupus erythematosus has not been reported following chlorthalidone administration.
6. Adverse Reactions/Side Effects
The following adverse reactions are described in more detail elsewhere in the label;
- Acute Kidney Injury [see Warnings and Precautions (5.1)]
- Electrolyte Abnormalities [see Warnings and Precautions (5.2)]
- Metabolic Disturbances [see Warnings and Precautions (5.3)]
The following adverse reactions have been observed with chlorthalidone, but there is not enough systematic collection of data to support an estimate of their frequency.
Gastrointestinal System Reactions: anorexia, gastric irritation, nausea, vomiting, cramping, diarrhea, constipation, jaundice (intrahepatic cholestatic jaundice), pancreatitis.
Central Nervous System Reactions: dizziness, paresthesias, headache.
Hematologic Reactions: leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia.
Dermatologic-Hypersensitivity Reactions: purpura, photosensitivity, rash, urticaria, necrotizing angiitis (vasculitis) (cutaneous vasculitis), Lyell’s syndrome (toxic epidermal necrolysis).
Cardiovascular Reaction: Orthostatic hypotension.
Other Adverse Reactions: muscle spasm, weakness, restlessness, impotence, xanthopsia.
7. Drug Interactions
Effect of chlorthalidone on other drugs
Insulin requirements in diabetic patients may be increased, decreased or unchanged. Higher dosage of oral hypoglycemic agents may be required.
Chlorthalidone may increase the responsiveness to tubocurarine.
Chlorthalidone may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
Lithium renal clearance is reduced by chlorthalidone, increasing the risk of lithium toxicity. Monitor serum lithium levels during concomitant use.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data over decades from observational studies and reports with chlorthalidone use in pregnant women have not identified a drug-associated risk of major birth defects or miscarriage. However, adverse fetal outcomes, including fetal or neonatal jaundice, thrombocytopenia, hypoglycemia, and electrolyte abnormalities have been reported following maternal use of thiazide diuretics (see Clinical Considerations). Chlorthalidone should not be used as first-line therapy to treat hypertension in pregnancy. Advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 12.5 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and stillbirth.
Fetal/Neonatal Adverse Reactions
Thiazides can cross the placenta, and concentrations reached in the umbilical vein approach those in the maternal plasma. Thiazides, like other diuretics, can cause placental hypoperfusion. Use of thiazides during pregnancy is associated with a risk of fetal or neonatal jaundice, thrombocytopenia, hypoglycemia, and electrolyte abnormalities. Thiazides do not prevent or alter the course of EPH (Edema, Proteinuria, Hypertension) gestosis (pre-eclampsia) and should not be used as first-line therapy to treat hypertension in pregnant women.
Animal Data
Reproduction studies have been performed in the rat and the rabbit and have revealed no evidence of harm to the fetus due to chlorthalidone. The available data do not allow the calculation of comparisons between the exposure of chlorthalidone observed in animal studies to the systemic exposure that would be expected in humans.
8.2 Lactation
Risk Summary
Chlorthalidone is present in human milk. There is no information regarding the effects of chlorthalidone on the breastfed infant or the effects on milk production. Because of the potential for chlorthalidone accumulation which may lead to serious adverse reactions in the breastfed infant (such as jaundice, thrombocytopenia, hyperglycemia, electrolyte abnormalities), advise patients that breastfeeding is not recommended during treatment with chlorthalidone.
8.5 Geriatric Use
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Chlorthalidone is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
10. Overdosage
Symptoms of acute overdosage include nausea, weakness, dizziness and disturbances of electrolyte balance. The oral LD50 of the drug in the mouse and the rat is more than 25,000 mg/kg body weight. The minimum lethal dose (MLD) in humans has not been established. There is no specific antidote but gastric lavage is recommended, followed by supportive treatment. Where necessary, this may include intravenous dextrose-saline with potassium, administered with caution.
11. Hemiclor Description
HEMICLOR (chlorthalidone) is an antihypertensive/diuretic supplied as 12.5 mg tablets for oral use. Chlorthalidone is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It is a racemic mixture of 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl) benzenesulfonamide, with the following structural formula:
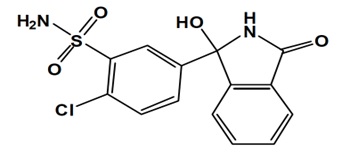
Molecular Formula: C14H11ClN2O4S Molecular Weight: 338.8 g/mol
Chlorthalidone is practically insoluble in water, soluble in acetone and in methanol, slightly soluble in 96% ethanol, practically insoluble in dichloromethane. It dissolves in diluted solutions of alkali hydroxides.
Each HEMICLOR tablet contains the following inactive ingredients: calcium stearate, colloidal silicon dioxide, microcrystalline cellulose, pregelatinized starch and sodium starch glycolate.
12. Hemiclor - Clinical Pharmacology
12.1 Mechanism of Action
Chlorthalidone is a long-acting oral diuretic with antihypertensive activity. Chlorthalidone produces diuresis with increased excretion of sodium and chloride. The site of action appears to be the cortical diluting segment of the ascending limb of Henle’s loop of the nephron. The diuretic effects of chlorthalidone lead to decreased extracellular fluid volume, plasma volume, cardiac output, total exchangeable sodium, glomerular filtration rate, and renal plasma flow. Although the mechanism of action of chlorthalidone and related drugs is not wholly clear, sodium and water depletion appear to provide a basis for its antihypertensive effect.
12.2 Pharmacodynamics
The diuretic action of chlorthalidone commences a mean of 2.6 hours after dosing and continues for up to 72 hours. Chlorthalidone produces dose-related reductions in serum potassium levels, elevations in serum uric acid and blood glucose, and it can lead to decreased sodium and chloride levels.
12.3 Pharmacokinetics
Distribution
Approximately 75 percent of the drug is bound to plasma proteins, 58 percent of the drug being bound to albumin. This is caused by an increased affinity of the drug to erythrocyte carbonic anhydrase. Data are not available regarding concentration of the drug in body fluids, degree of uptake by a particular organ or in the fetus, or passage across the blood-brain barrier.
Elimination
The mean plasma half-life of chlorthalidone is about 40 to 60 hours.
Metabolism
Data are not available regarding percentage of dose as unchanged drug and metabolites.
Excretion
Chlorthalidone is eliminated primarily as unchanged drug in the urine. Nonrenal routes of elimination have yet to be clarified.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity study has been conducted with chlorthalidone.
Mutagenesis: Chlorthalidone demonstrated no potential for mutagenic effects at non-cytotoxic concentrations and is considered not to pose a mutagenic risk to humans.
Impairment of Fertility: Chlorthalidone at a dosage of 100 mg/kg had no effect on fertility in rats.
14. Clinical Studies
The efficacy of a once daily dose of chlorthalidone 12.5 mg for the treatment of hypertension in adults is derived from a published randomized, double-blind, multinational, study (Sica et al, 2012)1 along with other supportive studies from the published literature.
- 1
- Sica D, Bakris GL, White WB, et al. J.Clin. Hypertens. 2012;14(5):284-292
16. How is Hemiclor supplied
HEMICLOR 12.5 mg tablets are supplied as white to off-white, modified rectangle shaped tablets debossed with “I” on one side and plain on the other side. HEMICLOR tablets are supplied as bottles of 30 tablets, 90 tablets, 100 tablets and 1,000 tablets.
NDC Number: 50742-285-30, Bottles of 30.
NDC Number: 50742-285-90, Bottles of 90.
NDC Number: 50742-285-01, Bottles of 100.
NDC Number: 50742-285-10, Bottles of 1,000.
Storage
Store at 20ºC to 25ºC (68ºF to 77ºF) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
17. Patient Counseling Information
Patients should inform their doctor if they have had an allergic reaction to chlorthalidone or other diuretics; kidney disease; gout; been taking lithium carbonate.
Patients should be cautioned to contact their physician if they experience any of the following symptoms of potassium loss: excess thirst, tiredness, drowsiness, restlessness, muscle pains or cramps, nausea, vomiting or increased heart rate or pulse.
Pregnancy
Advise a pregnant woman of the potential risk to a fetus. Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise females not to breastfeed during treatment with chlorthalidone [see Use in Specific Populations (8.2)].
Rx Only
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811
Rev. 03/2025
555800
| HEMICLOR
chlorthalidone tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ingenus Pharmaceuticals, LLC (833250017) |
| Registrant - PRM Pharma LLC (118256253) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ingenus Pharmaceuticals NJ, LLC | 964680206 | manufacture(50742-285) | |
Frequently asked questions
More about Hemiclor (chlorthalidone)
- Check interactions
- Compare alternatives
- Imprints, shape & color data
- Side effects
- Dosage information
- During pregnancy
- Drug class: thiazide diuretics
- Breastfeeding