Sunlenca Dosage
Generic name: LENACAPAVIR SODIUM 300mg
Dosage form: tablet, film coated
Drug class: Miscellaneous antivirals
Medically reviewed by Drugs.com. Last updated on Mar 26, 2025.
Adherence to Treatment Regimen
Prior to starting SUNLENCA, healthcare providers should carefully select patients who agree to the required every 6 month injection dosing schedule and counsel patients about the importance of adherence to scheduled SUNLENCA dosing visits and concomitant oral antiretroviral therapy to help maintain viral suppression and reduce the risk of viral rebound and potential development of resistance with missed doses.
Recommended Dosage
SUNLENCA can be initiated using one of the two recommended dosage regimens in Table 1 and Table 2 below. Maintenance dosing is administered by subcutaneous injection every 6 months regardless of the initiation regimen. Healthcare providers should determine the appropriate initiation regimen for the patient. SUNLENCA oral tablets may be taken with or without food.
| Treatment Time | |
|---|---|
|
|
| Dosage of SUNLENCA: Initiation | |
| Day 1 | 927 mg by subcutaneous injection (2 × 1.5 mL injections) 600 mg orally (2 × 300 mg tablets) |
| Day 2 | 600 mg orally (2 × 300 mg tablets) |
| Dosage of SUNLENCA: Maintenance | |
| Every 6 months (26 weeks) * +/-2 weeks | 927 mg by subcutaneous injection (2 × 1.5 mL injections) |
| Treatment Time | |
|---|---|
|
|
| Dosage of SUNLENCA: Initiation | |
| Day 1 | 600 mg orally (2 × 300 mg tablets) |
| Day 2 | 600 mg orally (2 × 300 mg tablets) |
| Day 8 | 300 mg orally (1 × 300 mg tablet) |
| Day 15 | 927 mg by subcutaneous injection (2 × 1.5 mL injections) |
| Dosage of SUNLENCA: Maintenance | |
| Every 6 months (26 weeks) * +/-2 weeks | 927 mg by subcutaneous injection (2 × 1.5 mL injections) |
Recommended Dosing Schedule for Missed Dose
Planned Missed Injections
During the maintenance period, if a patient plans to miss a scheduled 6-month injection visit by more than 2 weeks, SUNLENCA tablets may be taken for up to 6 months until injections resume. Refer to Table 3 below for the recommended dosage after planned missed injections.
| Time since Last Injection | Recommendation |
|---|---|
| 26 to 28 weeks | Maintenance oral dosage of 300 mg taken once every 7 days for up to 6 months. Resume the maintenance injection dosage within 7 days after the last oral dose. |
Unplanned Missed Injections
Patients who miss a scheduled injection visit should be clinically reassessed, including consideration of lenacapavir resistance testing, to ensure resumption of therapy remains appropriate. During the maintenance period, if more than 28 weeks have elapsed since the last injection and SUNLENCA tablets have not been taken, see Table 4 below for the recommended dosage after unplanned missed injections. Adherence to the injection dosing schedule is strongly recommended.
| Time since Last Injection | Recommendation |
|---|---|
| More than 28 weeks | Reinitiate with Option 1 (Table 1) or Option 2 (Table 2) and then continue with maintenance injection dosing. |
Preparation and Administration of Subcutaneous Injection
SUNLENCA injection is only for subcutaneous administration into the abdomen by a healthcare provider. Do NOT administer intradermally due to risk of serious injection site reactions.
Use aseptic technique. Visually inspect the solution in the vials and prepared syringe for particulate matter and discoloration prior to administration. SUNLENCA injection is a yellow solution. Do not use SUNLENCA injection if the solution is discolored or if it contains particulate matter. Once the solution is withdrawn from the vials, the subcutaneous injections should be administered as soon as possible.
There are two available injection kits, which differ only in how SUNLENCA injection is prepared (the components and associated method for withdrawal of the solution from the vials). Refer to the figures below for the relevant injection kit.
The injection kit components are for single use only. Two 1.5 mL injections are required for a complete dose.
Vial access device injection kit
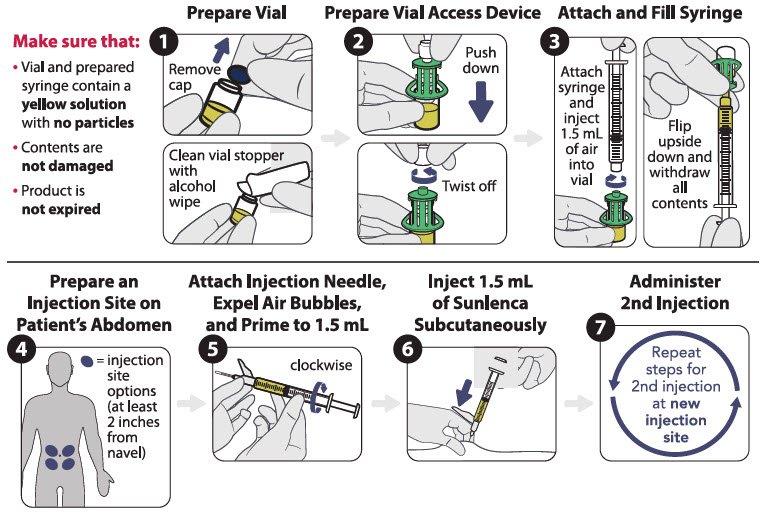
Figure 1 identifies the components for use in the administration steps for the vial access device injection kit, and the administration steps are provided in Figure 2. Use of a vial access device is required in this kit.
Figure 1 SUNLENCA Vial Access Device Injection Kit Components

Figure 2 SUNLENCA Injection Steps for Vial Access Device Injection Kit
Withdrawal needle injection kit
Figure 3 identifies the components for use in the administration steps for the withdrawal needle injection kit, and the administration steps are provided in Figure 4. The 18-gauge needle is for withdrawal only in this kit.
Figure 3 SUNLENCA Withdrawal Needle Injection Kit Components

Figure 4 SUNLENCA Injection Steps for Withdrawal Needle Injection Kit

More about Sunlenca (lenacapavir)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (2)
- Drug images
- Side effects
- During pregnancy
- FDA approval history
- Drug class: miscellaneous antivirals
- Breastfeeding
- En español
Patient resources
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.