Muse Prescribing Information
Package insert / product label
Generic name: alprostadil
Dosage form: urethral suppository
Drug classes: Impotence agents, Vasodilators
J Code (medical billing code): J0275 (OTH)
Medically reviewed by Drugs.com. Last updated on Jun 3, 2024.
On This Page
Muse Description
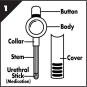
MUSE® (alprostadil) is a single-dose, medicated transurethral system for the delivery of alprostadil to the male urethra. Alprostadil is suspended in polyethylene glycol 1450 (as excipient) and is formed into a medicated pellet (micro-suppository measuring 1.4 mm in diameter by 3 mm or 6 mm in length) that resides in the tip of a translucent hollow applicator. MUSE is administered by inserting the applicator stem into the urethra after urination. The pellet containing alprostadil is delivered by depressing the applicator button (see Figure 1). The components of the delivery system are constructed of medical grade polypropylene. Each MUSE system is packaged in an individual foil pouch.
The active ingredient in MUSE is alprostadil, which is chemically identical to the naturally occurring eicosanoid, prostaglandin E1 (PGE1). The chemical name for alprostadil is prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-(11α,13E,15S)-(1R,2R,3R)-3-hydroxy-2-[(E)-(3S)-3-hydroxy-1-octenyl]-5-oxo-cyclopentane heptanoic acid, and the molecular weight is 354.49. The empirical formula is C20H34O5. The structural formula of alprostadil is represented below:
Alprostadil is a white to off-white crystalline powder with a melting point between 115° and 116°C. Its solubility at 35°C is 8000 mcg per 100 mL double-distilled water. The inactive ingredient in MUSE is polyethylene glycol 1450, USP. There are no other active agents or excipients in MUSE.
MUSE is available in 3 dosage strengths: 250 mcg, 500 mcg, and 1000 mcg.
Muse - Clinical Pharmacology
Mechanism of Action
Prostaglandin E1 is a naturally occurring acidic lipid that is synthesized from fatty acid precursors by most mammalian tissues and has a variety of pharmacologic effects. Human seminal fluid is a rich source of prostaglandins, including PGE1 and PGE2, and the total concentration of prostaglandins in ejaculate has been estimated to be approximately 100-200 mcg/mL. In vitro, alprostadil (PGE1) has been shown to cause dose-dependent smooth muscle relaxation in isolated corpus cavernosum and corpus spongiosum preparations. Additionally, vasodilation has been demonstrated in isolated cavernosal artery segments that were pre-contracted with either norepinephrine or prostaglandin F2α. When alprostadil was injected into the corpus cavernosum of pigtail monkeys in vivo, dose-dependent increases in cavernosal artery blood flow were observed.
In human studies using Doppler duplex ultrasonography, intraurethral administration of 500 mcg of MUSE resulted in an increase in cavernosal artery diameter and a 5- to 10-fold increase in peak systolic flow velocities. These results suggest that intraurethral alprostadil is absorbed from the urethra, transported throughout the erectile bodies by communicating vessels between the corpus spongiosum and corpora cavernosa, and able to induce vasodilation of the targeted vascular beds.
The vasodilatory effects of alprostadil on the cavernosal arteries and the trabecular smooth muscle of the corpora cavernosa result in rapid arterial inflow and expansion of the lacunar spaces within the corpora. As the expanded corporal sinusoids are compressed against the tunica albuginea, venous outflow through subtunical vessels is impeded and penile rigidity develops. This process is referred to as the corporal veno-occlusive mechanism.
The most notable systemic effects of alprostadil are vasodilation, inhibition of platelet aggregation, and stimulation of intestinal and uterine smooth muscle. Intravenous doses of 1 to 10 micrograms per kilogram of body weight lower blood pressure in mammals by decreasing peripheral resistance. Reflex increases in cardiac output and heart rate may accompany these effects.
Pharmacokinetics
About 80% of alprostadil administered by MUSE is absorbed within 10 minutes and is rapidly cleared from the systemic circulation by the lungs, leaving barely detectable systemic blood levels.
Absorption
MUSE is designed to deliver alprostadil directly to the urethral lining for transfer via the corpus spongiosum to the corpora cavernosa. Intraurethral administration of MUSE is preceded by urination, and the residual urine disperses the medicated pellet, permitting alprostadil to be absorbed by the urethral mucosa. The transurethral absorption of alprostadil after MUSE administration is biphasic. Initial absorption is rapid, with approximately 80% of an administered dose absorbed within 10 minutes. The mean time to the maximum plasma PGE1 concentration after a 1000 mcg intraurethral dose of MUSE is approximately 16 minutes.
In 10 normal human volunteers, endogenous PGE1 levels in the ejaculate averaged 31 mcg (range 0-161 mcg). In these same volunteers, an average of 123 mcg of additional PGE1 (range 30-369 mcg) was present in the ejaculate obtained 10 minutes after the highest dose (1000 mcg) of MUSE. The mean total endogenous PGE content (PGE1, PGE2, 19-OH-PGE1, and 19-OH-PGE2) of the ejaculate in these subjects was 444 mcg (range 0-1423 mcg).
Distribution
Following MUSE administration, alprostadil is absorbed from the urethral mucosa into the corpus spongiosum. A portion of the administered dose is transported to the corpora cavernosa through collateral vessels, while the remainder passes into the pelvic venous circulation through veins draining the corpus spongiosum. The half-life of alprostadil in humans is short, varying between 30 seconds and 10 minutes, depending on the body compartment in which it is measured and the physiological status of the subject. Nearly all of the alprostadil entering the central venous circulation is removed in a single pass through the lungs; thus peripheral venous plasma levels of PGE1 are low or undetectable (< 2 picograms/mL) after MUSE administration. The mean maximum plasma PGE1 concentration following intraurethral administration of the highest dose of MUSE (1000 mcg) was barely detectable (11.4 picograms/mL). In a study of 14 subjects, the plasma PGE1 level was shown to be undetectable within 60 minutes of MUSE administration in most subjects.
Metabolism
Alprostadil is rapidly metabolized locally by enzymatic oxidation of the 15-hydroxyl group to 15-keto-PGE1. The enzyme catalyzing this process has been isolated from many tissues in the lower genitourinary tract including the urethra, prostate, and corpus cavernosum. 15-keto-PGE1 retains little (1-2%) of the biological activity of PGE1. 15-keto-PGE1 is rapidly reduced at the C13-C14 position to form the most abundant metabolite in plasma, 13,14-dihydro,15-keto PGE1 (DHK-PGE1), which is biologically inactive. The majority of DHK-PGE1 is further metabolized to smaller prostaglandin remnants that are cleared primarily by the kidney and liver. Between 60% and 90% of PGE1 has been shown to be metabolized after 1 pass through the pulmonary capillary beds.
Excretion
After intravenous administration of tritium-labeled alprostadil in man, labeled drug disappears rapidly from the blood in the first 10 minutes, and by 1 hour radioactivity in the blood reaches a low level. The metabolites of alprostadil are excreted primarily by the kidney, with approximately 90% of an administered intravenous dose excreted in the urine within 24 hours of dosing. The remainder is excreted in the feces. There is no evidence of tissue retention of alprostadil or its metabolites following intravenous administration.
Pharmacokinetics in Special Populations
Pulmonary Disease
The near-complete pulmonary first-pass metabolism of PGE1 is the primary factor influencing the systemic pharmacokinetics of MUSE and is a reason that peripheral venous plasma levels of PGE1 are low or undetectable (< 2 picograms/mL) following MUSE administration. Patients with pulmonary disease therefore may have a reduced capacity to clear the drug. In patients with the adult respiratory distress syndrome (ARDS), pulmonary extraction of intravascularly administered alprostadil was reduced by approximately 15% compared to a control group of patients with normal respiratory function (66 ± 3.2% vs. 78 ± 2.4%).
Geriatrics
The effects of age on the pharmacokinetics of alprostadil have not been evaluated.
Clinical Studies
The efficacy of MUSE was evaluated in 7 placebo-controlled trials of various design in over 2500 patients with a history of erectile dysfunction of various etiologies. These trials assessed erectile function in the clinic and sexual intercourse in outpatient settings. In studies of sexual performance, patients were screened in the clinic, generally using doses of 125 mcg to 1000 mcg, for a satisfactory erectile response, then sent home with the selected dose or placebo for evaluation of sexual performance. Not all patients beginning titration had a successful dose and some patients could not tolerate MUSE, principally because of penile pain, so that the success rates in the studies described below must be understood to represent response rates only in patients who were successfully titrated.
In 2 identical multicenter, double-blind, placebo-controlled, parallel-group studies, 1511 patients with a mean 4-year history of erectile dysfunction and at least a 3-month history of no erections adequate for sexual intercourse without medical assistance, were enrolled and began dose titration in the clinic with doses between 125 mcg and 1000 mcg. 996 patients (66%) completed dose titration, achieved an erection sufficient for intercourse, and were randomized equally to placebo or active treatment and followed during at-home treatment for up to 3 months. 874 patients and partners completed 3 months of follow-up. About 10%, 20%, 30%, and 40% of patients were titrated to 125 mcg, 250 mcg, 500 mcg, and 1000 mcg, respectively. Couples on active therapy were more likely to have at least 1 successful sexual intercourse (65% vs. 19%) than were couples on placebo. Among patients who reported successful intercourse at least once with active treatment, approximately 7 of 10 times MUSE was used, patients reported successful sexual intercourse. Results were similar in patients with erectile dysfunction stemming from surgery or trauma, diabetes, vascular disease, or other etiologies, and were similar between racial sub-groups. In administrations resulting in sexual intercourse, the duration of erections sufficient for penetration was 6 minutes on placebo and 16 minutes on active drug. Successful therapy with MUSE was associated with improvement in the quality of life measures of “emotional well-being” for patients and “relationship with partner” for both patients and their female partners.
Indications and Usage for Muse
MUSE is indicated for the treatment of erectile dysfunction. Studies that established benefit demonstrated improvements in success rates for sexual intercourse compared with similarly administered placebo.
Contraindications
MUSE is contraindicated in men with any of the following:
- 1.
- Known hypersensitivity to alprostadil.
- 2.
- Abnormal penile anatomy: MUSE is contraindicated in patients with urethral stricture, balanitis (inflammation/infection of the glans of the penis), severe hypospadias and curvature, and in patients with acute or chronic urethritis.
- 3.
- Sickle cell anemia or trait, thrombocythemia, polycythemia, multiple myeloma: MUSE is contraindicated in patients who are prone to venous thrombosis or who have a hyperviscosity syndrome and are therefore at increased risk of priapism (rigid erection lasting 4 or more hours).
- 4.
- MUSE should not be used in men for whom sexual activity is inadvisable (see General Precautions).
- 5.
- MUSE should not be used for sexual intercourse with a pregnant woman unless the couple uses a condom barrier.
Warnings
Because of the potential for symptomatic hypotension and syncope, which occurred in 3% and 0.4%, respectively, of patients during in-clinic dosing, MUSE titration should be carried out under medical supervision. During post-marketing surveillance syncope occurring within one hour of administration has been reported. Patients should be cautioned to avoid activities, such as driving or hazardous tasks, where injury could result if hypotension or syncope were to occur after MUSE administration.
Precautions
General Precautions
- 1.
- A complete medical history and physical examination should be undertaken to exclude reversible causes of erectile dysfunction prior to the initiation of MUSE therapy. In addition, underlying disorders that might preclude the use of MUSE (see CONTRAINDICATIONS) should be sought.
- 2.
- Cardiovascular effects: During in-clinic dosing, patients should be monitored for symptoms of hypotension, and the lowest effective dose of MUSE should be prescribed.
- 3.
- Hematologic effects: Patients administering MUSE may be at risk of urethral abrasion resulting in minor bleeding or spotting. Patients on anticoagulant therapy or with bleeding disorders may be at higher risk of bleeding. Patients on anticoagulant therapy have been safely treated with MUSE; however, the risk/benefit ratio in these patients should be considered prior to prescribing MUSE.
- 4.
- Resumption of sexual activity: Sexual intercourse is considered a vigorous physical activity, and it increases heart rate as well as cardiac work. Physicians may want to examine the cardiac fitness of patients prior to treating erectile dysfunction.
- 5.
- Priapism and prolonged erection: In 2 identical multicenter, double-blind, placebo-controlled, parallel-group studies, priapism (rigid erection lasting 4 hours or longer) was reported in 2 of 1511 patients (0.13% of patients). Nevertheless, these reactions are a potential risk of pharmacologic therapy and can cause penile injury. Physicians should lower the dose or consider discontinuing MUSE treatment in any patient who develops priapism or prolonged erection.
- 6.
- Drug-Drug Interactions: Because there are low or undetectable (< 2 picograms/mL) amounts of alprostadil found in the peripheral venous circulation following MUSE administration, systemic drug-drug interactions with MUSE are unlikely. Although formal studies have not been conducted, the concomitant use of MUSE and anti-hypertensive medications may increase the risk of hypotension. It is therefore advised that caution be used in the administration of MUSE to individuals on anti-hypertensive medications. In addition, the presence of medications in the circulation that attenuate erectile function may influence the response to MUSE.
- 7.
- Drug-Device Interactions: Use of MUSE in patients with penile implants has not been studied.
Information for Patients
Patients should be informed that MUSE offers no protection from the transmission of sexually transmitted diseases. Patients and partners who use MUSE need to be counseled about the protective measures that are necessary to guard against the spread of sexually transmitted agents, including the human immunodeficiency virus (HIV).
Although unreported in clinical trials, there is the possibility that an overdosage of MUSE can cause priapism, a painful erection of the penis sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious and, if untreated, it can lead to permanent inability to have an erection. Patients who experience a prolonged erection should seek prompt medical attention.
Patients should be instructed how to administer MUSE. Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Information for Partners
Patients should be instructed to inform their partners that MUSE offers no protection from the transmission of sexually transmitted diseases. Counsel patients about the protective measures that are necessary to guard against the spread of sexually transmitted agents, including the human immunodeficiency virus (HIV). Human semen contains PGE1, but additional amounts may be present from MUSE administration (see CLINICAL PHARMACOLOGY). Partners who have experienced an extended period of sexual abstinence should be encouraged to seek advice from a health care professional prior to resuming sexual intercourse. The use of a water-based lubricant may facilitate vaginal penetration.
It is recommended that couples using MUSE employ adequate contraception if the female partner is of childbearing potential. There is no information on the effects on early pregnancy of PGE1 at the levels received by female partners. MUSE has no contraceptive properties. MUSE should not be used if the female partner is pregnant, unless the couple uses a condom barrier.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies of alprostadil have not been conducted. Alprostadil showed no evidence of mutagenicity in vitro in the Ames bacterial reverse mutation test, the unscheduled DNA synthesis assay in rat hepatocytes, or the Chinese hamster ovary forward gene mutation assay; nor was there evidence of mutagenicity in vivo in the mouse micronucleus assay. Alprostadil concentrations increased chromosomal aberrations above control incidence in the in vitro Chinese hamster ovary chromosomal aberration assay.
In dogs, sperm concentration, morphology, and motility were unaffected by daily intraurethral administration of up to 3000 mcg MUSE (alprostadil) for 13 weeks (200 mcg/kg/day or about 3.5 times the maximum recommended daily dose adjusted for body surface area). Alprostadil concentrations of 400 mcg/mL had no effect on human sperm motility or viability in vitro.
Pregnancy
Alprostadil has been shown to be embryotoxic (decreased fetal weight) when administered as a subcutaneous bolus to pregnant rats at doses as low as 500 mcg/kg/day. Doses of 2000 mcg/kg/day resulted in increased resorptions, reduced numbers of live fetuses, increased incidences of visceral and skeletal variations (primarily left umbilical artery and generalized reduction in ossification of the entire skeleton) and gross visceral and skeletal malformations (primarily edema, hydrocephaly, anophthalmia/microphthalmia, and skeletal anomalies). The latter dose produced maternal toxicity (ataxia, lethargy, diarrhea, and retarded body weight gain). When administered by continuous intravenous infusion, evidence of embryotoxicity (decreased fetal weight gain and increased incidence of hydroureter) was observed at 2000 mcg/kg/day, a dose that was also associated with a decrease in maternal weight gain. Intravaginal administration of up to 4000 mcg/day of MUSE (alprostadil) to pregnant rabbits (1100 mcg/kg/day or about 12.5 times the maximum recommended daily dose adjusted for body surface area) resulted in no evidence of harm to the fetus. MUSE should not be used for sexual intercourse with a pregnant woman unless the couple uses a condom barrier.
Adverse Reactions/Side Effects
In-Clinic Titration
In the 2 largest double-blind, parallel, placebo-controlled trials, 1511 patients received MUSE at least 1 time in the clinic setting. The most frequently reported adverse reactions during in-clinic titration included pain in the penis (36%), urethra (13%), or testes (5%). These reactions were most commonly reported as mild and transient, but about 7% of patients withdrew at this stage because of adverse reactions. Urethral bleeding/spotting and other minor abrasions to the urethra were reported in approximately 3% of patients. Symptomatic lowering of blood pressure (hypotension) occurred in 3% of patients; in addition, some lowering of blood pressure may occur without symptoms. Dizziness was reported in 4% of patients. Syncope (fainting) was reported by 0.4% of patients. (See WARNINGS).
Home Treatment
996 patients (66% of those who began titration) were studied during the home treatment portion of 2 Phase III placebo-controlled studies. Fewer than 2% of patients discontinued from these studies primarily because of adverse reactions. The following table summarizes the frequency of adverse reactions reported by patients using MUSE or placebo.
|
Event |
MUSE n = 486 |
Placebo n = 511 |
|
UROGENITAL SYSTEM | ||
|
Penile Pain |
32% |
3% |
|
Urethral Burning |
12% |
4% |
|
Minor Urethral Bleeding/Spotting |
5% |
1% |
|
Testicular Pain |
5% |
1% |
|
NERVOUS SYSTEM | ||
|
Dizziness |
2% |
< 1% |
|
BODY AS A WHOLE | ||
|
Flu Symptoms |
4% |
2% |
|
Headache |
3% |
2% |
|
Pain |
3% |
1% |
|
Accidental Injury |
3% |
2% |
|
Back Pain |
2% |
1% |
|
Pelvic Pain |
2% |
< 1% |
|
RESPIRATORY | ||
|
Rhinitis |
2% |
< 1% |
|
Infection |
3% |
2% |
Other drug-related side effects observed during in-clinic titration and home treatment include swelling of leg veins, leg pain, perineal pain, and rapid pulse, each occurring in < 2% of patients.
Female Partner Adverse Reactions
The most common adverse reactions reported by female partners during placebo-controlled clinical studies was vaginal burning/itching, reported by 5.8% of partners of patients on active vs. 0.8% of partners of patients on placebo. It is unknown whether this adverse reaction experienced by female partners was a result of the medication or a result of resuming sexual intercourse, which occurred much more frequently in partners of patients on active medication.
To report suspected adverse reactions, contact Meda Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX) or contact FDA at 1-800-FDA-1088, fax 1-800-FDA-0178 or online at www.fda.gov/medwatch.
Overdosage
Overdosage has not been reported with MUSE. Overdosage with MUSE may result in hypotension, persistent penile pain and possibly priapism (rigid erection lasting 4 or more hours). Priapism can result in permanent worsening of erectile function. Patients suspected of overdosage who develop these symptoms should be kept under medical supervision until systemic or local symptoms have resolved.
Muse Dosage and Administration
MUSE is a transurethral delivery system available in 3 dosage strengths: 250 mcg, 500 mcg, and 1000 mcg. MUSE should be administered as needed to achieve an erection. The onset of effect is within 5-10 minutes after administration. The duration of effect is approximately 30-60 minutes. However, the actual duration will vary from patient to patient. Instruct patients to straighten their penis to its full length when inserting the MUSE applicator. Each patient should be instructed by a medical professional on proper technique for administering MUSE prior to self-administration. The maximum frequency of use is no more than 2 systems per 24-hour period.
Initiation of Therapy
Dose titration should be administered under the supervision of a physician to test a patient's responsiveness to MUSE, to demonstrate proper administration technique (see detailed instructions for MUSE administration in Patient Information), and to monitor for evidence of hypotension (see WARNINGS). Patients should be individually titrated to the lowest dose that is sufficient for sexual intercourse. The lower dose of MUSE (250 mcg) is recommended for initial dosing. If necessary, the dose should be increased (or decreased) on separate occasions in a stepwise manner until the patient achieves an erection that is sufficient for sexual intercourse.
How is Muse supplied
MUSE is supplied in individual foil pouches containing one (1) single-dose system per pouch. MUSE is available in unit cartons containing six (6) systems. MUSE is available in the following 3 dosage strengths:
|
Dosage Strength |
NDC Numbers |
Identifying |
|
|
Carton |
Pouch |
Package Color |
|
|
250 mcg |
0037-8120-06 |
0037-8120-01 |
Green |
|
500 mcg |
0037-8130-06 |
0037-8130-01 |
Blue |
|
1000 mcg |
0037-8140-06 |
0037-8140-01 |
Burgundy |
Rx Only.
Storage and Handling
Store unopened foil pouches in a refrigerator at 2°C to 8°C (36°F to 46°F). Do not expose MUSE to temperatures above 30°C (86°F). MUSE may be kept by the patient at room temperature, 20°C to 25°C (68°F to 77°F), for up to 14 days before use.
For more information, call Meda Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
This product’s labeling may have been updated. For the most recent prescribing information, visit www.musehcp.com.
MUSE is a registered trademark of Meda AB, a Viatris company.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
Catalog Number: 05-10-00001X1
IN-1001-XX3
Revised: 5/2024
© 2024 Viatris Inc.
PATIENT INFORMATION
|
MUSE [myooz] (alprostadil) urethral suppository |
|
Read all of this Patient Information and Instructions for Use (see the other side of this Patient Information leaflet) before using MUSE as it contains important information. These materials provide visual instructions and more detailed information on how to use MUSE. |
|
What is MUSE? MUSE is a prescription medicine used to treat erectile dysfunction, commonly called impotence. Erectile dysfunction is the inability to attain or maintain an erection enough for sexual intercourse. |
|
Do not use MUSE if you:
|
|
Before you use MUSE, tell your healthcare provider about all of your medical conditions, including if:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How should I use MUSE?
|
|
What should I avoid while using MUSE?
|
|
What are the possible side effects of MUSE? The most serious side effects of MUSE include:
The most common side effects of MUSE include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of MUSE. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Meda Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX). |
|
How should I store MUSE?
Keep MUSE and all medicines out of the reach of children. |
|
General information about the safe and effective use of MUSE. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use MUSE for a condition for which it was not prescribed. Do not give MUSE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about MUSE that is written for health professionals. |
|
What are the ingredients in MUSE? Active Ingredient: alprostadil Inactive Ingredient: polyethylene glycol 1450, USP |
|
For more information, call Meda Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX). MUSE is a registered trademark of Meda AB, a Viatris company. Distributed by:
MEDA Somerset, New Jersey 08873-4120 Catalog Number: 05-10-00000X1 IS-1001-XX3 © 2024 Viatris Inc. |
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 05/2024
INSTRUCTIONS FOR USE
MUSE [myooz]
(alprostadil)
urethral suppository
This Instructions for Use contains information on how to insert MUSE.
This medicine has been prescribed to you only, do not pass it on to others.
MUSE is a single-dose applicator for the delivery of the urethral suppository into the male urethra for the treatment of erectile dysfunction.
The MUSE applicator contains a single dose in each foil pouch and the applicator is for 1 use only.
Storing MUSE
- •
- Store unopened MUSE foil pouches in a refrigerator between 36°F to 46°F (2°C to 8°C).
- •
- You may store MUSE at room temperature between 68°F to 77°F (20°C to 25°C), for up to 14 days before use.
- •
- Do not expose MUSE to temperatures above 86°F (30°C).
- •
- Keep MUSE and all medicines out of the reach of children.
Important Information You Need to Know Before Inserting MUSE
- •
- Always use MUSE exactly as your healthcare provider tells you to use it. Check with your healthcare provider or pharmacist if you are not sure.
- •
- Do not use more than 2 doses in 1 day (24-hour period).
- •
- If you use more MUSE than you should, contact your healthcare provider for advice.
- •
- You must insert MUSE within 10 minutes of urinating.
Preparing to Insert MUSE
Step 1. Urinate and gently shake your penis immediately before you insert the MUSE applicator.
- •
- Wash your hands with soap and water and dry them on a clean towel.
- •
- A moist urethra allows for easier insertion of the MUSE applicator. The urethral suppository dissolves in the small amount of urine that remains in the urethra after urination.
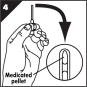
Step 2. Remove MUSE applicator from foil pouch.
- •
- Open the foil pouch by tearing fully across from the notched edge (Figure A).
- •
- Let the MUSE applicator slide out of the foil pouch onto your hand.
- •
- Save the foil pouch for throwing away the used MUSE applicator later.
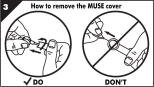
Step 3. Remove protective cover.
- •
- Hold the body of the MUSE applicator with your thumb and pointer finger (forefinger) (Figure B).
- •
- Twist the applicator body and pull out the MUSE applicator from the protective cover, being careful not to push in or pull out the button (Figure B).
- •
- Avoid touching the stem and tip.
- •
- Save the protective cover for throwing away the used MUSE applicator later.
Step 4. Inspect the MUSE applicator for the presence of the urethral suppository.
- •
- The MUSE applicator is see-through, and you should see the urethral suppository at the end of the tip. Make sure the urethral suppository is present before inserting the MUSE applicator into your urethra (Figure C).
- •
- Hold the MUSE applicator in the way which is most comfortable for you.
Inserting MUSE
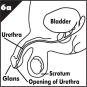
Refer to the Anatomy of the Penis figure below before proceeding to Step 5.
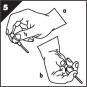
Step 5. Prepare penis for MUSE applicator insertion.
- •
- While sitting or standing, whichever is more comfortable for you, take several seconds to stretch your penis gently and slowly to its full length (Figure D).
- •
- Gently compress from the top to the bottom of the glans (Figure E). This straightens and opens the urethra.
Step 6. Insert MUSE applicator into urethra opening up to the collar (Figure F).
- •
- If you feel any discomfort or pulling sensation, pull back the MUSE applicator slightly and gently reinsert.
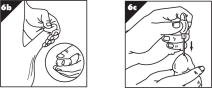
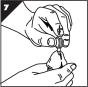
Step 7. Push down the button at the top of the MUSE applicator until it stops and hold for 5 seconds (Figure G).
- •
- It is important to do this to make sure that the urethral suppository is completely released.
- •
- Please note that the button will only move down slightly (there will be very little movement).
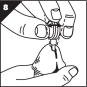
Step 8. Lift your finger off the button and gently move MUSE applicator side to side (Figure H).
- •
- This will separate the urethral suppository from the tip.
- •
- If you apply too much pressure you may scratch the lining of the urethra causing it to bleed.
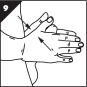
Step 9. Remove the MUSE applicator while keeping your penis upright (Figure I).
Step 10. Inspect the tip to see that the urethral suppository is no longer in the tip. Do not touch the stem.
- •
- If you notice the urethral suppository remaining in the tip or the stem of the MUSE applicator, repeat Step 6 through Step 9.
Step 11. Hold your penis upright and stretch it to its full length then roll your penis firmly between your hands for at least 10 seconds (Figure J).
- •
- This helps make sure the medicine covers the walls of your urethra.
Note: Your penis may feel warm and somewhat sensitive to the touch. This effect is normal and may last a few hours.
Disposing of (Throwing Away) MUSE
Step 12. Replace the protective cover on the MUSE applicator, place it in the opened foil pouch, and throw it away in your household trash.
- •
- Remember, MUSE is for single use only.
For more information, call Meda Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
MUSE is a registered trademark of Meda AB, a Viatris company.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
Catalog Number: 05-10-00000X
IFU-1001-XX2
© 2024 Viatris Inc.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved 05/2024
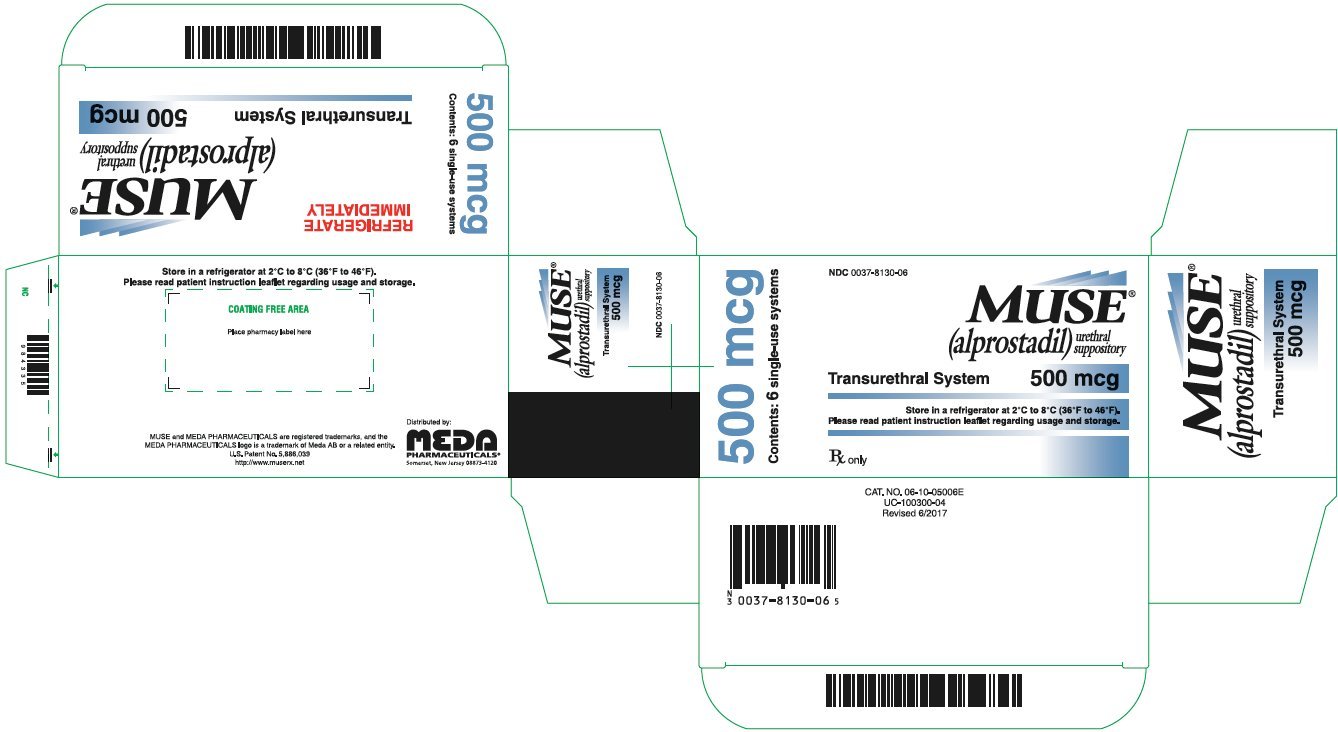
PRINCIPAL DISPLAY PANEL – 250 mcg
NDC 0037-8120-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 250 mcg
Please read the Patient Information and
Instructions for Use regarding usage.
Rx only
250 mcg
Contents: 6 single-use systems
REFRIGERATE
IMMEDIATELY
Store unopened MUSE foil pouches in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
You may store MUSE at room temperature, 68ºF to 77ºF (20ºC to 25ºC), for up to 14 days before use.
Do not expose MUSE to temperatures above 86ºF (30ºC).
Please read the Patient Information and Instructions for Use regarding usage.
Keep MUSE and all medicines out of reach of children.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
MUSE is a registered trademark of Meda AB, a Viatris company.
CAT. NO. 06-10-02506X
UC-100200-XX2
Revised X/202X
PRINCIPAL DISPLAY PANEL – 500 mcg
NDC 0037-8130-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 500 mcg
Please read the Patient Information and
Instructions for Use regarding usage.
Rx only
500 mcg
Contents: 6 single-use systems
REFRIGERATE
IMMEDIATELY
Store unopened MUSE foil pouches in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
You may store MUSE at room temperature, 68ºF to 77ºF (20ºC to 25ºC), for up to 14 days before use.
Do not expose MUSE to temperatures above 86ºF (30ºC).
Please read the Patient Information and Instructions for Use regarding usage.
Keep MUSE and all medicines out of reach of children.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
MUSE is a registered trademark of Meda AB, a Viatris company.
CAT. NO. 06-10-05006X
UC-100300-XX2
Revised X/202X
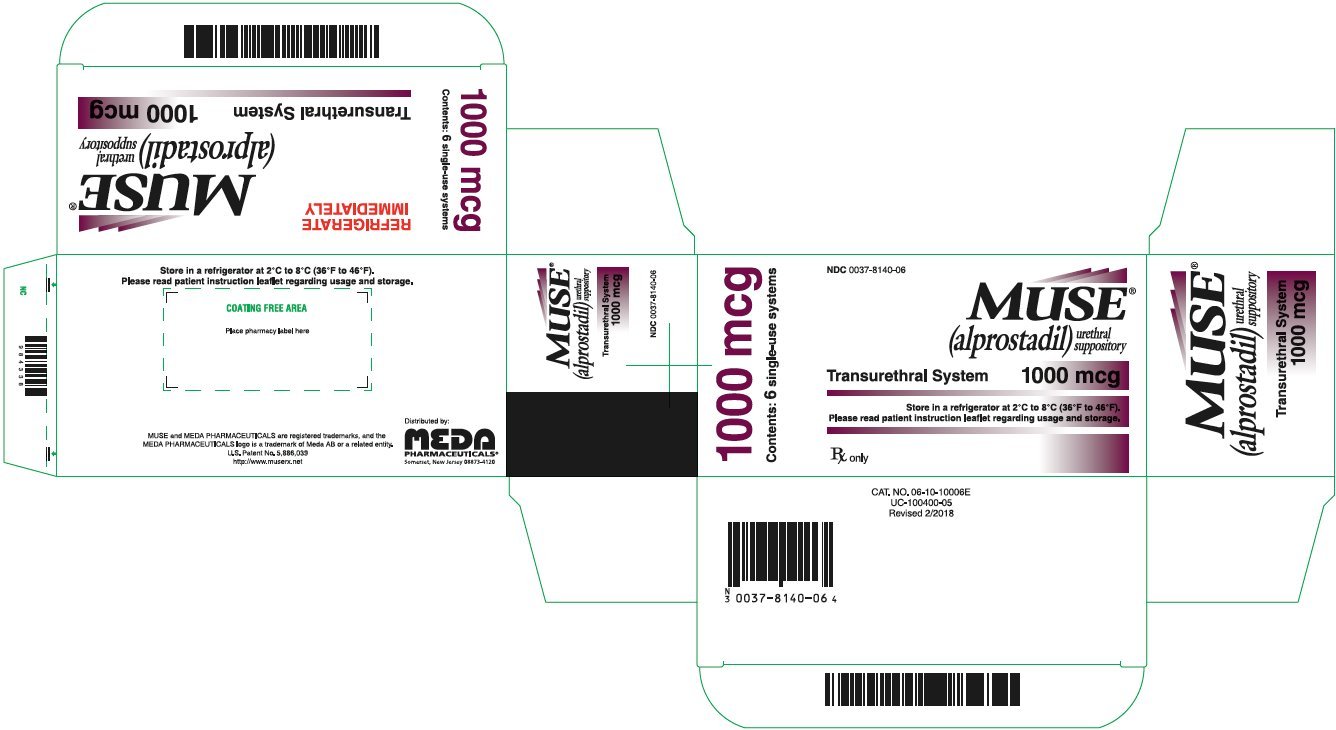
PRINCIPAL DISPLAY PANEL – 1000 mcg
NDC 0037-8140-06
MUSE®
(alprostadil)
urethral suppository
Transurethral System 1000 mcg
Please read the Patient Information and
Instructions for Use regarding usage.
Rx only
1000 mcg
Contents: 6 single-use systems
REFRIGERATE
IMMEDIATELY
Store unopened MUSE foil pouches in a refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
You may store MUSE at room temperature, 68ºF to 77ºF (20ºC to 25ºC), for up to 14 days before use.
Do not expose MUSE to temperatures above 86ºF (30ºC).
Please read the Patient Information and Instructions for Use regarding usage.
Keep MUSE and all medicines out of reach of children.
Distributed by:
MEDA
PHARMACEUTICALS®
Somerset, New Jersey 08873-4120
MUSE is a registered trademark of Meda AB, a Viatris company.
CAT. NO. 06-10-10006X
UC-100400-XX2
Revised X/202X
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| MUSE
alprostadil suppository |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Meda Pharmaceuticals Inc. (051229602) |
More about Muse (alprostadil)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (54)
- Side effects
- Dosage information
- During pregnancy
- Drug class: impotence agents
- En español
Patient resources
Professional resources
Other brands
Caverject, Edex, Caverject Impulse, Prostin VR Pediatric