Zenrelia (ilunocitinib tablets) (4.8 mg) (Canada)
This treatment applies to the following species: Company: Elanco
Company: Elanco
For Veterinary Use Only
Immunomodulator (Janus Kinase Inhibitor)
For Oral Use in Dogs Only
DIN 02552051
DIN 02552086
DIN 02552094
DIN 02552108
Description
Zenrelia (ilunocitinib) is a yellow, scored, oblong film-coated tablet that can be divided into equal halves. Zenrelia is available in four strengths with each strength containing 4.8 mg, 6.4 mg, 8.5 mg and 15 mg of ilunocitinib per tablet, respectively.
Zenrelia (ilunocitinib tablets) (4.8 mg) Indications
Zenrelia is indicated for the control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age.
Directions For Use
The dose of Zenrelia (ilunocitinib tablet) is 0.6 to 0.8 mg/kg of body weight. Zenrelia is to be administered orally, once daily, with or without food.
Table 1. Dosing Chart
|
Body Weight (kg) |
Number of Tablets to be Administered |
|||
|
4.8 mg Tablets |
6.4 mg Tablets |
8.5 mg Tablets |
15 mg Tablets |
|
|
3 - 4 |
0.5 |
|
|
|
|
4.1 - 5.3 |
|
0.5 |
|
|
|
5.4 - 6.5 |
|
|
0.5 |
|
|
6.6 - 8 |
1 |
|
|
|
|
8.1 - 10.6 |
|
1 |
|
|
|
10.7 - 14.1 |
|
|
1 |
|
|
14.2 - 16 |
|
1.5 |
|
|
|
16.1 - 19.5 |
|
|
1.5 |
|
|
19.6 - 24.9 |
|
|
|
1 |
|
25 - 28.3 |
|
|
2 |
|
|
28.4 - 37.4 |
|
|
|
1.5 |
|
37.5 - 49.9 |
|
|
|
2 |
|
50 - 62.4 |
|
|
|
2.5 |
|
62.5 - 74.9 |
|
|
|
3 |
|
≥ 75 |
Administer the appropriate combination of tablet strengths |
|||
Contraindications
Zenrelia should not be administered to:
● Dogs with serious infections or those with evidence of malignant neoplasia, demodicosis or immune suppression
● Breeding, pregnant or lactating dogs
CAUTIONS
Based on the results of the vaccine response study, there is a risk of a delayed humoral immune response to vaccinations and a risk of vaccine-associated disease with modified live vaccination in dogs treated with Zenrelia. To reduce these risks:
● Dogs should be up to date on vaccinations prior to starting treatment with Zenrelia.
● Dogs should discontinue Zenrelia for at least 28 days prior to vaccination and withhold Zenrelia for at least 28 days after vaccination.
● A longer washout period may be prudent to ensure an adequate response to killed vaccination for dogs on chronic therapy (see Target Animal Safety).
The benefits and risks of Zenrelia to the individual patient should be assessed prior to use.
Zenrelia modulates the immune system. Dogs should be monitored for the development of infections because Zenrelia may increase susceptibility to opportunistic infections, including demodicosis, interdigital furunculosis, coccidiosis, and pneumonia, or exacerbation of subclinical or uncomplicated infections (see Target Animal Safety and Adverse Reactions). Active infections should be resolved whenever possible prior to starting therapy with Zenrelia.
Zenrelia may cause a progressive or persistently decreased hematocrit, hemoglobin, and/or red blood cell count without a corresponding increase in absolute reticulocyte count (see Target Animal Safety). Periodic monitoring of the complete blood count is recommended when dogs are on treatment long-term.
Neoplastic conditions (benign and malignant) were observed in dogs treated with Zenrelia during clinical studies (see Adverse Reactions). Dogs receiving Zenrelia should be monitored for signs of neoplasia.
Zenrelia is not for use in dogs less than 12 months of age (see Target Animal Safety). The safe use of Zenrelia has not been evaluated in dogs less than 3 kg body weight.
The safe use of Zenrelia has not been evaluated in combination with glucocorticoids, cyclosporine, or other systemic immunosuppressive agents.
WARNINGS
Keep out of reach of children. Not for use in humans. Wash hands immediately after handling tablets. In case of accidental ingestion, seek medical attention immediately.
ADVERSE REACTIONS
In a masked field study assessing efficacy and safety of Zenrelia for the control of atopic dermatitis in dogs, 181 Zenrelia-treated dogs and 87 placebo-treated dogs were evaluated for safety up to 112 days. In the placebo group, 48% of dogs exited the study by Day 29, and 67% exited by Day 112, compared to 8% and 22% for Zenrelia-treated dogs, respectively.
In another masked field study assessing efficacy and safety of Zenrelia for the control of pruritus associated with allergic dermatitis in dogs, 206 Zenrelia-treated dogs and 100 placebo-treated dogs were evaluated for safety up to 112 days. In the placebo group, 39% of dogs exited the study by Day 28, and 84% exited by Day 112, compared to 9% and 50% for Zenrelia-treated dogs, respectively.
Adverse reactions seen at a frequency ≥ 2% during these field studies are summarized in Table 2 below.
Table 2. Adverse reactions in the field studies through Day 112
|
Adverse Reaction |
Atopic Dermatitis Study |
Allergic Dermatitis Study |
||
|
Zenrelia N = 181 (%) |
Placebo N = 87 (%) |
Zenrelia N = 206 (%) |
Placebo N = 100 (%) |
|
|
Pruritus |
43 (23.8%) |
22 (25.3%) |
21 (10.2%) |
9 (9%) |
|
Vomiting or nausea |
38 (21%) |
14 (16%) |
32 (16%) |
13 (13%) |
|
Diarrhea |
34 (18.8%) |
9 (10.3%) |
26 (12.6%) |
5 (5%) |
|
Lethargy |
21 (11.6%) |
9 (10.3%) |
24 (11.7%) |
7 (7%) |
|
Otitis externa |
19 (10.5%) |
20 (23%) |
7 (3.4%) |
5 (5%) |
|
Dermatitis and eczema |
19 (10.5%) |
12 (13.8%) |
8 (3.9%) |
11 (11%) |
|
Eye disorders1 |
19 (10%) |
6 (6.9%) |
6 (2.9%) |
0 |
|
Anorexia |
17 (9.4%) |
7 (8%) |
10 (4.9%) |
3 (3%) |
|
Dermal mass or lipoma |
15 (8.2%) |
4 (4.5%) |
2 (1%) |
0 |
|
Cough or sneezing |
14 (7.7%) |
2 (2.2%) |
9 (4.4%) |
0 |
|
Neutropenia or Leucopenia |
12 (6.6%) |
2 (2.3%) |
1 (0.5%) |
0 |
|
Urinary tract infection |
10 (5.5%) |
2 (2.3%) |
5 (2.4%) |
1 (1%) |
|
Tooth or gingival disorder |
10 (5.5%) |
4 (4.6%) |
4 (1.9%) |
0 |
|
Bacterial skin infection |
9 (5%) |
8 (9.2%) |
2 (1%) |
4 (4%) |
|
Trauma |
9 (5%) |
3 (3.4%) |
5 (2.4%) |
3 (3%) |
|
Other skin disorders2 |
8 (4.4%) |
9 (10%) |
11 (5.3%) |
7 (7%) |
|
Lameness |
8 (4.4%) |
2 (2.3%) |
5 (2.4%) |
0 |
|
Otorrhea |
7 (3.9%) |
6 (6.9%) |
6 (2.9%) |
1 (1%) |
|
Elevated liver enzymes |
7 (3.9%) |
2 (2.3%) |
5 (2.4%) |
0 |
|
Weight gain |
7 (3.9%) |
0 |
2 (1%) |
0 |
|
Erythema |
6 (3.3%) |
3 (3.4%) |
6 (2.9%) |
5 (5%) |
|
Other ear disorders3 |
6 (3.3%) |
4 (4.6%) |
3 (1.5%) |
4 (4%) |
|
Alopecia |
5 (2.8%) |
7 (8%) |
6 (2.9%) |
5 (5%) |
|
Other abnormal test result |
5 (2.8%) |
1 (1.1%) |
3 (1.5%) |
0 |
|
Skin lesion or dermal thickening |
5 (2.7%) |
4 (4.6%) |
4 (1.9%) |
2 (2%) |
|
Dermal cysts |
4 (2.2%) |
0 |
0 |
1 (1%) |
|
Elevated serum alkaline phosphate |
4 (2.2%) |
0 |
5 (2.4%) |
0 |
|
Elevated total bilirubin |
4 (2.2%) |
0 |
1 (0.5%) |
0 |
|
Elevated serum lipids |
4 (2.2%) |
0 |
5 (2.4%) |
0 |
|
Polydipsia |
4 (2.2%) |
2 (2.3%) |
7 (3.4%) |
2 (2%) |
|
Abnormal behaviour |
4 (2.2%) |
0 |
1 (0.5%) |
0 |
|
Flatulence, bloating and distension |
3 (1.7%) |
0 |
2 (1%) |
3 (3%) |
|
Systemic disorder |
2 (1.1%) |
2 (2.3%) |
1 (0.5%) |
1 (1%) |
|
Anemia |
1 (0.6%) |
1 (1.1%) |
0 |
2 (2%) |
|
Urine abnormalities |
1 (0.6%) |
2 (2.3%) |
3 (1.5%) |
1 (1%) |
|
Inappropriate urination |
1 (0.6%) |
2 (2.3%) |
1 (0.5%) |
1 (1%) |
N = Number of dogs enrolled; NOS = not otherwise specified
1 Includes VeDDRA terms: epiphora, eye disorder NOS, eye redness and periorbital edema
2 Includes VeDDRA terms: pigmentation disorder, desquamation, skin textural change and skin disorder NOS
3 Includes VeDDRA terms: pinnal irritation and other ear disorder
In the atopic dermatitis field study, twelve Zenrelia-treated dogs withdrew from the study early due to an adverse event, nine of which were considered likely related to Zenrelia treatment. These adverse events included episodes of vomiting, leukopenia, neutropenia, worsening of pre-existing lymphocytosis, enlargement of a non-resolving histiocytoma, eyelid mass with bacterial blepharitis, otitis interna with vestibular disease, urinary tract infection, and upper respiratory infection. Five placebo dogs withdrew from the study early due to an adverse event (i.e., lethargy, worsening of pre-existing lymphocytosis, occurrence of nystagmus, skin infection, and teat infection).
One Zenrelia-treated dog was euthanized one month after study completion following abdominal rupture of a metastatic splenic and hepatic hemangiosarcoma. Another Zenrelia-treated dog experienced a moderate neutropenia on Day 28 associated with a pre-existing subclinical urinary tract infection (UTI) that progressed into a clinical UTI. The neutrophil count normalized seven days later while receiving Zenrelia, prior to exiting the study to receive antibiotics.
In the allergic dermatitis field study, seven Zenrelia-treated dogs withdrew from the study early due to adverse reactions, four of which were considered likely related to Zenrelia treatment. These events included vomiting, lethargy, soft stool, neutropenia, increased liver enzymes, coughing, and wheezing. Four placebo-treated dogs also withdrew from the study early due to an adverse reaction (i.e., splenic hemangiosarcoma, restlessness, abdominal pain, lethargy, and vomiting).
One Zenrelia-treated dog experienced vomiting, dyspnea, depression, fever, abdominal discomfort, and slightly worsened azotemia on Day 4. The dog had a pre-existing elevated creatinine level. Another Zenrelia-treated dog experienced vomiting, diarrhea, and lethargy starting on Day 1 and mild neutropenia on Day 3 that resolved five days later.
To report suspected adverse drug events or for technical assistance, contact Elanco Canada Limited at 1-800-265-5475.
SCIENTIFIC INFORMATION
Active Ingredient
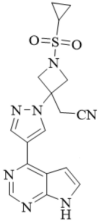
The active ingredient in Zenrelia tablets is ilunocitinib, a synthetic Janus Kinase (JAK) inhibitor of the pyrimidines class of JAK inhibitors. The chemical name is 2-[1-cyclopropylsulfonyl-3-[4-(7H-pyrrolo[2,3-d] pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile, with a molecular formula of C17H17N7O2S and a molecular weight of 383.43 g/mol. The chemical structure of ilunocitinib is provided below:
Clinical Pharmacology
Pharmacodynamics
Ilunocitinib is a non-selective Janus kinase (JAK) inhibitor that inhibits the function of a variety of pruritogenic, pro-inflammatory and allergy related cytokines that are dependent upon these enzymes. Ilunocitinib has a high potency for JAK1, JAK2, and tyrosine kinase 2 (TYK2) inhibition. Ilunocitinib is not a corticosteroid or an antihistamine.
Pharmacokinetics
Ilunocitinib is rapidly absorbed and excreted via the biliary/fecal route after oral administration in dogs. Following a single oral or intravenous administration of ilunocitinib at 0.8 mg/kg, the mean oral bioavailability based on area under the curve from the time of dosing to the last quantifiable plasma (AUClast) was 80% in the fed state and 58% in the fasted state (n=16). The maximum plasma concentration (Cmax) and AUC were 120% and 45% higher, respectively, in the fed state as compared to the fasted state. The mean systemic clearance following intravenous administration is 437 mL/h/kg with a mean terminal half-life of 4.42 hours (n=8). The mean volume of distribution is 1580 mL/kg.
Target Animal Safety:
Margin of Safety Study
Zenrelia was administered to 40 (8 dogs per group) healthy, 11 to 12-month-old, fed Beagle dogs, weighing between 5.5 kg and 12.4 kg. Tablets were administered once daily at 0X, 1X, 2X, 3X and 5X the maximum recommended dose of 0.8 mg/kg for 6 months. Control dogs were sham-dosed. Zenrelia-related clinical observations included a dose-dependent increase in the frequency and severity of interdigital furunculosis (cysts), with or without discharge on one or more paws, swollen and/or scabbing paws, and paw skin thickening and/or discoloration.
Zenrelia-related hemogram findings included a dose-dependent minimal to moderate decrease in hematocrit (HCT), hemoglobin (HGB), and red blood cell (RBC) count without a corresponding increase in absolute reticulocyte count. Other Zenrelia-related findings included a minimal to mild decrease in mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentrations (MCHC), and eosinophil counts. Abnormal clinical pathology observations included minimal to moderate increases in fibrinogen concentrations, total protein, C-reactive protein, and globulin, and decreases in albumin, albumin/globulin ratio and calcium levels. There were no Zenrelia-related effects on lymphocytes, monocytes, and basophils. Two dogs in the 5X group had minimally lower myeloid:erythroid ratios consistent with a physiological bone marrow response to the lower red blood cell mass despite no apparent effect on absolute reticulocytes. Zenrelia-related macroscopic and microscopic pathology changes included decreased prostate gland weights in the 5X group males and interdigital papillomas and/or dermatitis/furunculosis, predominantly in the 5X group. One dog in the 5X group had enlarged and mildly reactive draining lymph nodes associated with interdigital furunculosis. One dog in the 3X group had a papilloma on each paw with fragments of Demodex canis, and a follicular cyst within the markedly inflamed dermis.
Vaccine Response Study
Zenrelia was administered to 16 healthy, 10-month-old, vaccine naïve Beagle dogs (8 dogs per group), weighing between 5.8 kg and 15.4 kg. Tablets were administered once daily at 0 mg/kg (control) and 2.4 mg/kg (3X the maximum recommended dose of 0.8 mg/kg) for 89 days. Control dogs were placebo-dosed. Dogs were administered a multivalent modified live virus (MLV) vaccine containing canine distemper virus (CDV), canine parvovirus (CPV), canine adenovirus-2 (CAV-2), and canine parainfluenza virus (CPiV) on Days 28 and 60 and a killed rabies virus (RV) vaccine on Day 60. The primary endpoint of the study was predefined serum titer thresholds considered indicative of protective immunity on Day 88. On Day 54, one dog administered Zenrelia was euthanized due to lethargy, depression, poor body condition, and weakness. Histopathology revealed findings consistent with infectious canine hepatitis (ICH), caused by canine adenovirus, secondary to Zenrelia-induced immunosuppression. On Day 52, another dog administered Zenrelia was euthanized due to lethargy, depression, poor body condition, and weakness. Necropsy revealed findings consistent with a colonic intussusception potentially related to Cystoisospora canis infection, which was secondary to immune suppression.
Clinical signs observed in Zenrelia-treated dogs included poor body condition, pale mucous membranes, lethargy, diarrhea, vomiting, weight loss, decreased appetite, and depression likely due to C. canis infection secondary to Zenrelia-induced immunosuppression in seven of the eight dogs. No dogs in the control group were diagnosed with C. canis. One dog in the control group had diarrhea. Administration of Zenrelia was also associated with interdigital cysts, lameness, and thickening and crusting of the margins of the ears. Clinical pathology findings in Zenrelia-treated dogs included decreases in hematocrit, hemoglobin, and red blood cell counts with a corresponding increase in absolute reticulocyte count, and decreases in total serum protein, albumin and globulins. Immunophenotyping analysis indicated a decrease in total T lymphocytes, helper T lymphocytes, and cytotoxic T lymphocytes and an increase in B lymphocytes.
All eight control dogs demonstrated an adequate humoral response to the multivalent MLV (for CDV, CPV, and CAV-2) and killed RV vaccines on Day 88. All six remaining dogs receiving Zenrelia achieved an adequate humoral response on Day 88 to the canine adenovirus type-2 (CAV-2) and canine parvovirus (CPV) vaccination. Of the remaining six dogs treated with Zenrelia, adequate humoral response on Day 88 was not achieved in four dogs for the rabies virus (RV) vaccination and one dog for the canine distemper virus (CDV) vaccination. Both the control group and the Zenrelia treatment group each had two dogs that did not achieve an adequate humoral response on Day 88 to the canine parainfluenza (CPiV) vaccination. Three of the four dogs that failed to achieve an adequate humoral response on Day 88 to the RV vaccination achieved an adequate titer level on Day 116 (28 days after discontinuing Zenrelia). The one dog that failed to achieve an adequate humoral response on Day 88 to the CDV vaccination also failed to achieve an adequate response on Day 88 to the RV vaccination.
Target Animal Efficacy:
Control of Atopic Dermatitis
A masked, placebo-controlled field study was conducted at 25 veterinary clinics in the United States and in Canada, enrolling 268 client-owned dogs (> 3.1 kg, > 12 months of age) diagnosed with atopic dermatitis. Dogs were randomized to once daily treatment with Zenrelia at 0.6 - 0.8 mg/kg or placebo, at a ratio of 2:1 respectively. Other medications that could affect the evaluation of efficacy were not allowed during the study. Treatment success for each dog was defined as a ≥ 50% reduction from baseline in owner-assessed pruritus scores on the Pruritus Visual Analog Scale (PVAS) or a ≥ 50% reduction from baseline in veterinarian-assessed skin lesion scores on the Canine Atopic Dermatitis Extent and Severity Index version 4 (CADESI-4) on Day 28. To be enrolled in the study, a dog must have had at least moderate pruritus (PVAS score ≥ 6.0 out of 10), and at least mild skin lesions (CADESI-4 score ≥ 25 out of 180). The proportion of dogs in the Zenrelia-treated group that were treatment successes was greater and significantly different compared to the placebo group on Day 28 (Table 3 below).
Table 3. Estimated proportion of dogs achieving treatment success (≥ 50% reduction from baseline on PVAS or CADESI-4 score)
|
Treatment Group |
Estimated Proportion of Success* |
P-value |
|
Zenrelia (N = 172) |
0.83 |
< 0.001 |
|
Placebo (N = 77) |
0.31 |
* Based on back-transformed least squares means
N = Number of dogs
The Zenrelia-treated group had a higher proportion of dogs with a ≥ 50% reduction from baseline in owner-assessed PVAS scores and veterinarian-assessed CADESI-4 scores, compared to placebo, at all timepoints (Days 14, 28, 56, 84 and 112).
Control of Pruritus Associated with Allergic Dermatitis
A masked, placebo-controlled field study was conducted at 15 veterinary clinics in the US, enrolling 306 client-owned dogs (> 3 kg, > 12 months of age) diagnosed with allergic dermatitis. Dogs were randomized to once daily treatment with Zenrelia at 0.6 - 0.8 mg/kg or placebo, at a ratio of 2:1 respectively. Other medications that could affect the evaluation of efficacy were not allowed during the study. Treatment success for each dog was defined as a ≥ 50% reduction from baseline in owner-assessed pruritus scores on the Pruritus Visual Analog Scale (PVAS) on at least 5 out of the first 7 days of treatment. To be enrolled in the study, a dog must have had at least moderate pruritus (PVAS score ≥ 6.0 out of 10). The proportion of dogs in the Zenrelia-treated group that were treatment successes was greater and significantly different compared to the placebo group on Day 7 (see Table 4 below).
Table 4. Estimated proportion of dogs achieving treatment success (≥ 50% reduction from baseline on PVAS scores on at least 70% of Days 1-7 (at least 5 of 7 days))
|
Treatment Group |
Estimated Proportion of Success* |
P-value |
|
Zenrelia (N = 193) |
0.25 |
0.006 |
|
Placebo (N = 91) |
0.08 |
* Based on back-transformed least squares means
N = Number of dogs
STORAGE INFORMATION
Store at room temperature between 15°C to 25°C. Excursions permitted between 5°C to 40°C.
PRESENTATION/PACKAGE TYPES
Zenrelia is available in scored tablets in four strengths: 4.8 mg per tablet, 6.4 mg per tablet, 8.5 mg per tablet and 15 mg per tablet. Each strength is available in 10 and 30 count blister packages, and 90 count bottles. Not all pack sizes may be marketed.
MANUFACTURED FOR
Elanco Canada Limited, 1919 Minnesota Court, Suite 401, Mississauga, Ontario L5N 0C9
1-800-265-5475
Date: September 2024
Zenrelia, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2024 Elanco or its affiliates.
26Sep2024
CPN: 1231246.0
1919 MINNESOTA COURT, SUITE 401, MISSISSAUGA, ON, L5N 0C9
| Customer Service: | 800-265-5475 | |
| Fax: | 519-821-7831 | |
| Website: | www.elanco.ca | |
| Email: | elancocanadacustomerservice@elancoah.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
