Xylased 20 (Canada)
This treatment applies to the following species: Company: Modern Veterinary Therapeutics
Company: Modern Veterinary Therapeutics
(Xylazine Injection, Mfr. Std.)
Sterile
20 mg/mL
Sedative and Analgesic for Use In Dogs, Cats and Cattle
VETERINARY USE ONLY
DIN 02540894
Description
Xylased 20™ (xylazine) is supplied in 50 mL multiple-dose vials as a sterile solution. Each mL contains 20 mg xylazine (as xylazine hydrochloride) as an active ingredient, 1 mg methylparaben as a preservative, 4.3 mg sodium chloride, sterile water; and hydrochloric acid for pH adjustment.
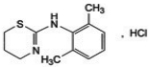
The chemical name is N-(2,6-dimethylphenyl)-5,6-dihydro-4H-1,3-thiazin-2-amine hydrochloride.
Chemical Formula: C12H17ClN2S. Molecular weight: 256.8 g/moL.
Chemical Structure:
Xylased 20 Indications
Dogs and Cats: Xylased 20 should be used in dogs and cats when it is desirable to produce a state of sedation accompanied by a shorter period of analgesia. Xylased 20 has been used successfully as follows:
1. Diagnostic procedures - examination of the mouth and ears, abdominal palpation, rectal palpation, vaginal examination, catheterization of the bladder and radiographic examinations.
2. Orthopedic procedures, such as application of casting materials and splints.
3. Dental procedures.
4. Minor surgical procedures of short duration such as debridement, removal of cutaneous neoplasms and suturing of lacerations.
5. To calm and facilitate handling of fractious animals.
6. Therapeutic medication for sedation and relief of pain following injury or surgery.
7. Major surgical procedures:
a. When used as a preanesthetic to general anesthesia.
b. When used in conjunction with local anesthetics.
Cattle: Xylased 20 is indicated in cattle to produce a state of sedation accompanied by a shorter period of analgesia. It has been used successfully as follows:
1. Diagnostic procedures - oral, vaginal and rectal examinations, as an aid in the collection of biopsies or blood samples and radiographic examinations.
2. Orthopedic procedures, such as application of casting materials and splints.
3. Dental procedures.
4. Minor surgical procedures of short duration such as debridement of wounds, dehorning, castration and suturing of skin lacerations.
5. Major surgical procedures when used in conjunction with local and epidural anesthetics - suturing of lacerations of the teat and udder, surgery of the penis and sheath, caesarean sections, hernia repairs, digital amputations and eye enucleations.
6. Hoof trimming and handling of fractious animals.
Dosage and Administration
Dogs and Cats:
1. Intravenously - 0.5 mL/9 kg body weight (1.1 mg/kg or 0.5 mg/lb)
Intramuscularly or subcutaneously - 1.0 mL/9 kg body weight (2.2 mg/kg or 1.0 mg/lb). In large dogs (over 22.7 kg/50 lbs) a dosage of 1.1 mg/kg (0.5 mg/lb) administered intramuscularly may provide sufficient sedation and/or analgesia for most procedures.
Since vomiting may occur (see ADVERSE REACTIONS), fasting for 6-24 hours prior to the use of Xylased 20 may reduce the incidence; the I.V. route results in the least vomiting.
Following injection of Xylased 20, the animal should be allowed to rest quietly until the full effect has been reached.
These dosages produce sedation which is usually maintained for 1 to 2 hours and analgesia which lasts for 15 to 30 minutes.
2. Preanesthetic to local anesthesia: Xylased 20 at the recommended dosages can be used in conjunction with local anesthetics such as procaine or lidocaine.
3. Preanesthetic to general anesthesia: Xylazine, at the recommended dosage rates, produces an additive effect to central nervous system depressants such as pentobarbital sodium, thiopental sodium and thiamylal sodium. Therefore, the dosage of such compounds should be reduced and administered to the desired effect. In general, only 1/3 to 1/2 of the calculated dosage of the barbiturates will be needed to produce a surgical plane of anesthesia. Post anesthetic or emergence excitement has not been observed in animals pre-anesthetized with xylazine.
Xylazine has been used successfully as a preanesthetic agent for pentobarbital sodium, thiopental sodium, thiamylal sodium, nitrous oxide, ether, halothane, and methoxyflurane anesthesia.
Cattle: Intramuscularly - Range of 0.25 to 0.75 mL/45 kg body weight (Equivalent to 0.11 to 0.33 mg/kg or 0.05 to 0.15 mg/lb).
Ruminants are more sensitive to xylazine than are other species in which the drug is indicated, and thus a much smaller dose is required per unit body weight to produce the desired effect. The dosage of Xylased 20 in the bovine species needed to achieve the desired effect varies between animals, depending largely upon the temperament of the individual animal. Quieter or more docile cattle will require a smaller dose to achieve the same effect. Xylased 20 will often make the animal recumbent especially at the higher dose rates. Following injection of Xylased 20 the animal should be allowed to rest quietly until the full effect has been reached.
Within the recommended dosage range, a range of side effects can be achieved depending on the dose given. Low doses of xylazine produce a sedation and limited dermal analgesia while larger doses produce sedation, muscle relaxation and analgesia along with a sleep like state. This sleep like state, in conjunction with the sedation, analgesia and muscle relaxation described, produce recumbency and a true anesthesia like condition under which many procedures may be carried out with or without local anesthesia. Even high doses will not eliminate pain in the claws and lower limbs.
After intramuscular injection of xylazine, the onset of sedation and analgesia follows in less than 10 minutes along with some incoordination. The duration of sedation and analgesia along with the ability to stand depends on the dose given. Duration of sedation and analgesia will vary from 30 minutes with low doses to 2 to 3 hours with higher doses.
Within the recommended dosage range, Xylased 20 can be used in conjunction with local anesthetics such as procaine and lidocaine. Many procedures may be carried out using Xylased 20 alone especially at the higher dose rate.
Contraindications
Dogs and Cats: Xylased 20 is not recommended for use in pregnant cats.
Do not use Xylased 20 in conjunction with tranquilizers.
Cattle: Do not use in pregnant animals as studies have not been completed to show its safety in all stages of pregnancy. Premature parturition and retained placenta have been reported in a limited number of cases where xylazine was administered during the last trimester of pregnancy.
Do not use Xylased 20 in conjunction with tranquilizers.
CAUTIONS:
Dogs and Cats: Until more definitive studies are completed, Xylased 20 is not recommended for use in pregnant cats.
Careful consideration should be given before administering to dogs or cats with significantly depressed respiration, severe pathologic heart disease, advanced liver or kidney disease, severe endotoxic or traumatic shock.
Since an additive effect results from the use of xylazine and the barbiturate compounds, it should be used with caution with these central nervous system depressants. Products known to produce respiratory depression or apnea, such as thiamylal sodium, should be given at a reduced dosage and, when injected intravenously, should be administered SLOWLY.
When intravenous administration is desired, avoid perivascular injection in order to achieve the desired effect. Studies have shown negligible evidence of tissue irritation, however, following perivascular injection of xylazine.
Following the use of Xylased 20, veterinarians and attendants should continue to use care and appropriate animal handling techniques, since conscious animals, although sedated, are arousable and capable of inflicting personal injury. Bradycardia and an arrhythmia in the form of incomplete atrioventricular block have been reported following xylazine administration. Although clinically the importance of this effect is questioned, a standard dose of atropine given prior to or following Xylased 20 will greatly decrease the incidence.
Cattle: Careful consideration should be given before administering to cattle with significantly depressed respiration, severe pathologic heart disease, advanced liver or kidney disease, severe endotoxic or traumatic shock.
Special precautions should be taken when administering during warm environmental conditions as HYPERTHERMIA may occur. Proper aftercare must be provided in those cases. Always provide cool shade during the recovery period.
Lateral recumbency is to be avoided during recovery due to increasing the possibilities of bloat, regurgitation and/or aspiration. Sternal recumbency is the appropriate recovery position. A 24-hour fast prior to injection will also reduce the incidence of bloat.
Following the use of Xylased 20, veterinarians and attendants should continue to use care and appropriate animal handing techniques, since conscious animals, although sedated, are capable of inflicting personal injury.
Warnings
This drug is for use in Dogs, Cats and Cattle only.
Treated cattle must not be slaughtered for use in food for at least 3 days after the latest treatment with this drug. Milk taken from treated animals during treatment and within 48 hours after the latest treatment must not be used as food.
Xylazine is an alpha2-adrenergic agonist with sedative, some analgesic and muscle relaxant properties.
Symptoms after absorption in humans may include dose-dependent respiratory depression, bradycardia, hypotension, dry mouth, and hyperglycemia. Ventricular arrhythmias have also been reported.
Do not eat, drink or smoke while handling the product.
Strictly avoid self-injection, oral intake and any contact with skin, eyes or mucosa. In the case of accidental contact, wash exposed skin or eyes abundantly with water. If symptoms occur, seek medical advice. In the case of accidental oral intake or self-injection, seek the advice of a physician and show the package insert but DO NOT DRIVE.
If pregnant women handle the product, special caution should be observed not to self-inject as uterine contractions and decreased fetal blood pressure may occur after accidental systemic exposure.
Dispose the unused drug or waste materials safely in accordance with the provincial/municipal guidelines.
Keep out of reach of children.
Adverse Reactions
Dogs and Cats: Emesis occurs occasionally in dogs and frequently in cats, soon after the administration of xylazine. When observed, emesis usually occurs only a single time, after which there is no further emetic effect.
The use of antiemetics may delay this phenomenon.
The occurrence of emesis may be considered a desirable effect when Xylased 20 is administered as a preanesthetic to general anesthesia.
Xylased 20 used at recommended dosage levels may occasionally cause slight muscle tremors, bradycardia with partial A-V heart block and a reduced respiratory rate. Should excessive respiratory depression occur following the use of Xylased 20, administer respiratory stimulants and provide artificial respiration.
Movement in response to sharp auditory stimuli may be observed.
Increased urination may occur in cats following the use of Xylased 20.
Cattle: Xylased 20 used at recommended dosage levels may occasionally cause slight muscle tremors, bradycardia and a reduced respiratory rate. Temporary salivation, diuresis and ruminal stasis may be observed during the period of sedation. A transient, self-limiting diarrhea may occur 24 to 48 hours following administration.
To report suspected adverse drug events or for technical assistance, contact Modern Veterinary Therapeutics, LLC at 1-888-590-9839.
SAFETY: Cattle: Xylazine has been tolerated in cattle at 10 times the recommended dose. However, doses of this magnitude produced muscle tremors and long periods of sedation; careful surveillance was necessary during the recovery period.
Pharmacology
Xylazine is a potent sedative and analgesic as well as muscle relaxant. Its sedative and analgesic activity is related to central nervous system depression. Its muscle-relaxant effect is based on inhibition of the intraneural transmission of impulses in the central nervous system. The principal pharmacological activities develop within 10 to 15 minutes after intramuscular injection and within 3 to 5 minutes following intravenous administration.
A sleeplike state, the depth of which is dose-dependent, is usually maintained for 1 to 2 hours, while analgesia lasts from 15 to 30 minutes. The centrally acting muscle relaxant effect causes relaxation of the skeletal musculature, complementing sedation and analgesia.
In animals under the influence of xylazine, the respiratory rate is reduced as in natural sleep. Following treatment with xylazine, the heart rate is decreased and a transient change in the conductivity of the cardiac muscle may occur, as evidenced by a partial atrioventricular block. This resembles the atrioventricular block often observed in normal animals. Intravenous administration of xylazine causes a transient rise in blood pressure, followed by a slight decrease.
Xylazine has no effect on blood clotting time or other hematologic parameters. In limited tests, xylazine has been tolerated in dogs and cats at 10 times the recommended dose. However, doses of this magnitude produce muscle tremors, emesis and long periods of sedation.
Storage
Store between 15°C - 25°C. Discard any remainder 28 days after broaching. The stopper should not be punctured more than 25 times.Manufactured for:
Modern Veterinary Therapeutics, LLC, Sunrise, Florida 33326 USA
info@modernveterinarytherapeutics.com
Imported by:
Modern Veterinary Therapeutics Inc., 261065 Wagon Wheel Way, Bay 3, Balzac (Rocky View County), AB T4A 0T5
Rev. 12/23
CPN: 1354038.0
261065 WAGON WHEEL WAY, ROCKY VIEW COUNTY, AB, T4A 0T5
| Telephone: | 407-852-8039 | |
| Toll-Free: | 888-590-9839 | |
| Website: | www.modernveterinarytherapeutics.com | |
| Email: | info@modernveterinarytherapeutics.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
