Tulieve Injectable Solution
This treatment applies to the following species: Company: Norbrook
Company: Norbrook
(tulathromycin injection)
Antibiotic
100 mg of tulathromycin/mL
For use in beef cattle (including suckling calves), non-lactating dairy cattle (including dairy calves), veal calves, and swine. Not for use in female dairy cattle 20 months of age or older.
Tulieve Injectable Solution Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Description
Tulieve® Injectable Solution is a ready-to-use sterile parenteral preparation containing tulathromycin, a semi-synthetic macrolide antibiotic of the subclass triamilide. Each mL of Tulieve contains 100 mg of tulathromycin, 500 mg propylene glycol, 19.2 mg citric acid and 5 mg monothioglycerol. Sodium hydroxide or hydrochloric acid may be added to adjust pH.
Tulieve consists of an equilibrated mixture of two isomeric forms of tulathromycin in a 9:1 ratio. Structures of the isomers are shown below.
Figure 1.
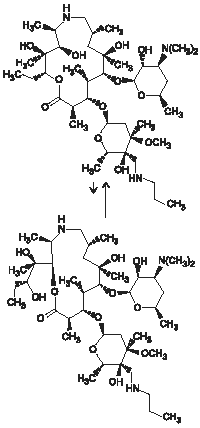
The chemical names of the isomers are (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R) - 13 - [[2,6 - dideoxy - 3 - C - methyl - 3 - O - methyl - 4 - C - [(propylamino)methyl] - α - L - ribo - hexopyrano - syl]oxy] - 2 - ethyl - 3,4,10 - trihydroxy - 3,5,8,10,12,14 - hexamethyl - 11 - [[3,4,6 - trideoxy - 3 - (dimethylamino) - β - D - xylo - hexopyranosyl] - oxy] - 1 - oxa - 6 - azacyclopentadecan - 15 - one and (2R,3R,6R, 8R,9R,10S,11S,12R) - 11 - [[2,6 - dideoxy - 3 - C - methyl - 3 - O - methyl - 4 - C - [(propylamino)methyl] - α - L - ribo - hexopyrano - syl]oxy] - 2 - [(1R,2R) - 1,2 - dihydroxy - 1 - methylbutyl] - 8 - hydroxy - 3,6,8,10,12 - pentamethyl - 9 - [[3,4,6 - trideoxy - 3 - (dimethylamino) - β - D - xylo - hexopyranosyl]oxy] - 1 - oxa - 4 - azacyclotridecan - 13 - one, respectively.
Tulieve Injectable Solution Indications
Beef And Non-lactating Dairy Cattle
BRD-Tulieve Injectable Solution is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis; and for the control of respiratory disease in cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis.
IBK-Tulieve Injectable Solution is indicated for the treatment of infectious bovine keratoconjunctivitis (IBK) associated with Moraxella bovis.
Foot Rot-Tulieve Injectable Solution is indicated for the treatment of bovine foot rot (interdigital necrobacillosis) associated with Fusobacterium necrophorum and Porphyromonas levii.
Suckling Calves, Dairy Calves, And Veal Calves
BRD-Tulieve Injectable Solution is indicated for the treatment of BRD associated with M. haemolytica, P. multocida, H. somni, and M. bovis.
Swine
Tulieve Injectable Solution is indicated for the treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis, and Mycoplasma hyopneumoniae; and for the control of SRD associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, and Mycoplasma hyopneumoniae in groups of pigs where SRD has been diagnosed.
Tulieve Injectable Solution Dosage And Administration
Cattle
Inject subcutaneously as a single dose in the neck at a dosage of 2.5 mg/kg (1.1 mL/100 lb) body weight (BW). Do not inject more than 10 mL per injection site.
Table 1. Tulieve Cattle Dosing Guide
|
Animal Weight (Pounds) |
Dose Volume (mL) |
|
100 |
1.1 |
|
200 |
2.3 |
|
300 |
3.4 |
|
400 |
4.5 |
|
500 |
5.7 |
|
600 |
6.8 |
|
700 |
8.0 |
|
800 |
9.1 |
|
900 |
10.2 |
|
1000 |
11.4 |
Swine
Inject intramuscularly as a single dose in the neck at a dosage of 2.5 mg/kg (0.25 mL/22 lb) BW. Do not inject more than 2.5 mL per injection site.
Table 2. Tulieve Swine Dosing Guide
|
Animal Weight (Pounds) |
Dose Volume (mL) |
|
15 |
0.2 |
|
30 |
0.3 |
|
50 |
0.6 |
|
70 |
0.8 |
|
90 |
1.0 |
|
110 |
1.3 |
|
130 |
1.5 |
|
150 |
1.7 |
|
170 |
1.9 |
|
190 |
2.2 |
|
210 |
2.4 |
|
230 |
2.6 |
|
250 |
2.8 |
|
270 |
3.1 |
|
290 |
3.3 |
Contraindications
The use of Tulieve Injectable Solution is contraindicated in animals previously found to be hypersensitive to the drug.
Warnings
FOR USE IN ANIMALS ONLY.
NOT FOR HUMAN USE.
KEEP OUT OF REACH OF CHILDREN.
NOT FOR USE IN CHICKENS OR TURKEYS.
 |
Residue WarningsCattleCattle intended for human consumption must not be slaughtered within 18 days from the last treatment. This drug is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. SwineSwine intended for human consumption must not be slaughtered within 5 days from the last treatment. |
 |
Precautions
Cattle
The effects of tulathromycin injection on bovine reproductive performance, pregnancy, and lactation have not been determined. Subcutaneous injection can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
Swine
The effects of tulathromycin injection on porcine reproductive performance, pregnancy, and lactation have not been determined. Intramuscular injection can cause a transient local tissue reaction that may result in trim loss of edible tissue at slaughter.
Adverse Reactions
Cattle
In one BRD field study, two calves treated with tulathromycin injection at 2.5 mg/kg BW exhibited transient hypersalivation. One of these calves also exhibited transient dyspnea, which may have been related to pneumonia.
Swine
In one field study, one out of 40 pigs treated with tulathromycin injection at 2.5 mg/kg BW exhibited mild salivation that resolved in less than four hours.
Post Approval Experience
The following adverse events are based on post approval adverse drug experience reporting. Not all adverse events are reported to the FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data. The following adverse events are listed in decreasing order of reporting frequency in cattle: Injection site reactions and anaphylaxis/anaphylactoid reactions. For a complete listing of adverse reactions for tulathromycin injection reported to the CVM see: www.fda.gov/reportanimalae.
Clinical Pharmacology
At physiological pH, tulathromycin (a weak base) is approximately 50 times more soluble in hydrophilic than hydrophobic media. This solubility profile is consistent with the extracellular pathogen activity typically associated with the macrolides.1 Markedly higher tulathromycin concentrations are observed in the lungs as compared to the plasma. The extent to which lung concentrations represent free (active) drug was not examined. Therefore, the clinical relevance of these elevated lung concentrations is undetermined.
Although the relationship between tulathromycin and the characteristics of its antimicrobial effects has not been characterized, as a class, macrolides tend to be primarily bacteriostatic, but may be bactericidal against some pathogens.2 They also tend to exhibit concentration independent killing; the rate of bacterial eradication does not change once serum drug concentrations reach 2 to 3 times the minimum inhibitory concentration (MIC) of the targeted pathogen. Under these conditions, the time that serum concentrations remain above the MIC becomes the major determinant of antimicrobial activity. Macrolides also exhibit a post-antibiotic effect (PAE), the duration of which tends to be both drug and pathogen dependent. In general, by increasing the macrolide concentration and the exposure time, the PAE will increase to some maximal duration. Of the two variables, concentration and exposure time, drug concentration tends to be the most powerful determinant of the duration of PAE.
Tulathromycin is eliminated from the body primarily unchanged via biliary excretion.
1 Carbon, C. 1998. Pharmacodynamics of Macrolides, Azalides, and Streptogramins: Effect on Extracellular Pathogens. Clin. Infect. Dis., 27:28-32.
2 Nightingale, C.J. 1997. Pharmacokinetics and Pharmacodynamics of Newer Macrolides. Pediatr. Infect. Dis. J., 16:438-443.
Cattle
Following subcutaneous administration into the neck of feeder calves at a dosage of 2.5 mg/kg BW, tulathromycin is rapidly and nearly completely absorbed. Peak plasma concentrations generally occur within 15 minutes after dosing and product relative bioavailability exceeds 90%. Total systemic clearance is approximately 170 mL/hr/kg. Tulathromycin distributes extensively into body tissues, as evidenced by volume of distribution values of approximately 11 L/kg in healthy ruminating calves.3 This extensive volume of distribution is largely responsible for the long elimination half-life of this compound [approximately 2.75 days in the plasma (based on quantifiable terminal plasma drug concentrations) versus 8.75 days for total lung concentrations (based on data from healthy animals)]. Linear pharmacokinetics are observed with subcutaneous doses ranging from 1.27 mg/kg BW to 5.0 mg/kg BW. No pharmacokinetic differences are observed in castrated male versus female calves.
3 Clearance and volume estimates are based on intersubject comparisons of 2.5 mg/kg BW administered by either subcutaneous or intravenous injection.
Swine
Following intramuscular administration to feeder pigs at a dosage of 2.5 mg/kg BW, tulathromycin is completely and rapidly absorbed (Tmax ~0.25 hour). Subsequently, the drug rapidly distributes into body tissues, achieving a volume of distribution exceeding 15 L/kg. The free drug is rapidly cleared from the systemic circulation (CLsystemic = 187 mL/hr/kg). However, it has a long terminal elimination half-life (60 to 90 hours) owing to its extensive volume of distribution. Although pulmonary tulathromycin concentrations are substantially higher than concentrations observed in the plasma, the clinical significance of these findings is undetermined. There are no gender differences in swine tulathromycin pharmacokinetics.
Microbiology
Cattle
Tulathromycin has demonstrated in vitro activity against Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis, four pathogens associated with BRD; against Moraxella bovis associated with IBK; and against Fusobacterium necrophorum and Porphyromonas levii associated with bovine foot rot.
The MICs of tulathromycin against indicated BRD and IBK pathogens were determined using methods recommended by the Clinical and Laboratory Standards Institute (CLSI, M31-A2). The MICs against foot rot pathogens were also determined using methods recommended by the CLSI (M11-A6). All MIC values were determined using the 9:1 isomer ratio of this compound.
BRD-The MICs of tulathromycin were determined for BRD isolates obtained from calves enrolled in therapeutic and at-risk field studies in the U.S. in 1999. In the therapeutic studies, isolates were obtained from pre-treatment nasopharyngeal swabs from all study calves, and from lung swabs or lung tissue of saline-treated calves that died. In the at-risk studies, isolates were obtained from nasopharyngeal swabs of saline-treated non-responders, and from lung swabs or lung tissue of saline-treated calves that died. The results are shown in Table 3.
IBK-The MICs of tulathromycin were determined for Moraxella bovis isolates obtained from calves enrolled in IBK field studies in the U.S. in 2004. Isolates were obtained from pre-treatment conjunctival swabs of calves with clinical signs of IBK enrolled in the tulathromycin injection and saline-treated groups. The results are shown in Table 3.
Foot Rot-The MICs of tulathromycin were determined for Fusobacterium necrophorum and Porphyromonas levii obtained from cattle enrolled in foot rot field studies in the U.S. and Canada in 2007. Isolates were obtained from pretreatment interdigital biopsies and swabs of cattle with clinical signs of foot rot enrolled in the tulathromycin injection and saline-treated groups. The results are shown in Table 3.
Table 3. Tulathromycin minimum inhibitory concentration (MIC) values* for indicated pathogens isolated from field studies evaluating BRD and IBK in the U.S. and from foot rot field studies in the U.S. and Canada.
|
Indicated pathogen |
Date Isolated |
No. of isolates |
MIC50** (µg/mL) |
MIC90** (µg/mL) |
MIC range (µg/mL) |
|
Mannheimia haemolytica |
1999 |
642 |
2 |
2 |
0.5 to 64 |
|
Pasteurella multocida |
1999 |
221 |
0.5 |
1 |
0.25 to 64 |
|
Histophilus somni |
1999 |
36 |
4 |
4 |
1 to 4 |
|
Mycoplasma bovis |
1999 |
43 |
0.125 |
1 |
≤ 0.063 to > 64 |
|
Moraxella bovis |
2004 |
55 |
0.5 |
0.5 |
0.25 to 1 |
|
Fusobacterium necrophorum |
2007 |
116 |
2 |
64 |
≤ 0.25 to > 128 |
|
Porphyromonas levii |
2007 |
103 |
8 |
128 |
≤ 0.25 to > 128 |
*The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
**The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
Swine
In vitro activity of tulathromycin has been demonstrated against Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis, and Mycoplasma hyopneumoniae.
The MICs of tulathromycin against indicated SRD pathogens were determined using methods recommended by the Clinical and Laboratory Standards Institute (CLSI, M31-A and M31-A3). MICs for Haemophilus parasuis were determined using Veterinary Fastidious Medium and were incubated up to 48 hours at 35 to 37°C in a CO2-enriched atmosphere. All MIC values were determined using the 9:1 isomer ratio of this compound. Isolates obtained in 2000 and 2002 were from lung samples from saline-treated pigs and non-treated sentinel pigs enrolled in Treatment of SRD field studies in the U.S. and Canada. Isolates obtained in 2007 and 2008 were from lung samples from saline-treated and tulathromycin injection-treated pigs enrolled in the Control of SRD field study in the U.S. and Canada. The results are shown in Table 4.
Table 4. Tulathromycin minimum inhibitory concentration (MIC) values* for indicated pathogens isolated from field studies evaluating SRD in the U.S. and Canada.
|
Indicated pathogen |
Date Isolated |
No. of isolates |
MIC50** (µg/mL) |
MIC90** (µg/mL) |
MIC range (µg/mL) |
|
Actinobacillus pleuropneumoniae |
2000-2002 2007-2008 |
135 88 |
16 16 |
32 16 |
16 to 32 4 to 32 |
|
Haemophilus parasuis |
2000-2002 |
31 |
1 |
2 |
0.25 to > 64 |
|
Pasteurella multocida |
2000-2002 2007-2008 |
55 40 |
1 1 |
2 2 |
0.5 to > 64 ≤ 0.03 to 2 |
|
Bordetella bronchiseptica |
2000-2002 |
42 |
4 |
8 |
2 to 8 |
*The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
**The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
Effectiveness
Cattle
BRD-In a multi-location field study, 314 calves with naturally occurring BRD were treated with tulathromycin injection. Responses to treatment were compared to saline-treated controls. A cure was defined as a calf with normal attitude/activity, normal respiration, and a rectal temperature of ≤104°F on Day 14. The cure rate was significantly higher (P ≤ .05) in tulathromycin injection-treated calves (78%) compared to saline-treated calves (24%). There were two BRD-related deaths in the tulathromycin injection-treated calves compared to nine BRD-related deaths in the saline-treated calves.
Fifty-two tulathromycin injection-treated calves and 27 saline-treated calves from the multi-location field BRD treatment study had Mycoplasma bovis identified in cultures from pre-treatment nasopharyngeal swabs. Of the 52 tulathromycin injection-treated calves, 37 (71.2%) calves were categorized as cures and 15 (28.8%) calves were categorized as treatment failures. Of the 27 saline-treated calves, 4 (14.8%) calves were categorized as cures and 23 (85.2%) calves were treatment failures.
A Bayesian meta-analysis was conducted to compare the BRD treatment success rate in young calves (calves weighing 250 lbs or less and fed primarily a milk-based diet) treated with tulathromycin injection to the success rate in older calves (calves weighing more than 250 lbs and fed primarily a roughage and grain-based diet) treated with tulathromycin injection. The analysis included data from four BRD treatment effectiveness studies conducted for the approval of tulathromycin injection in the U.S. and nine contemporaneous studies conducted in Europe. The analysis showed that the BRD treatment success rate in young calves was at least as good as the BRD treatment success rate in older calves. As a result, tulathromycin injection is considered effective for the treatment of BRD associated with M. haemolytica, P. multocida, H. somni, and M. bovis in suckling calves, dairy calves, and veal calves.
In another multi-location field study with 399 calves at high risk of developing BRD, administration of tulathromycin injection resulted in a significantly reduced incidence of BRD (11%) compared to saline-treated calves (59%). Effectiveness evaluation was based on scored clinical signs of normal attitude/activity, normal respiration, and a rectal temperature of ≤104°F on Day 14. There were no BRD-related deaths in the tulathromycin injection-treated calves compared to two BRD-related deaths in the saline-treated calves. Fifty saline-treated calves classified as non-responders in this study had Mycoplasma bovis identified in cultures of post-treatment nasopharyngeal swabs or lung tissue.
Two induced infection model studies were conducted to confirm the effectiveness of tulathromycin injection against Mycoplasma bovis. A total of 166 calves were inoculated intratracheally with field strains of Mycoplasma bovis. When calves became pyrexic and had abnormal respiration scores, they were treated with either tulathromycin injection (2.5 mg/kg BW) subcutaneously or an equivalent volume of saline. Calves were observed for signs of BRD for 14 days post-treatment, then were euthanized and necropsied. In both studies, mean lung lesion percentages were statistically significantly lower in the tulathromycin injection-treated calves compared with saline-treated calves (11.3% vs. 28.9%, P = 0.0001 and 15.0% vs. 30.7%, P < 0.0001).
IBK-Two field studies were conducted evaluating tulathromycin injection for the treatment of IBK associated with Moraxella bovis in 200 naturally-infected calves. The primary clinical endpoint of these studies was cure rate, defined as a calf with no clinical signs of IBK and no corneal ulcer, assessed on Days 5, 9, 13, 17, and 21. Time to improvement, defined as the first day on which a calf had no clinical signs of IBK in both eyes, provided that those scores were maintained at the next day of observation, was assessed as a secondary variable. At all time points, in both studies, the cure rate was significantly higher (P < 0.05) for tulathromycin injection-treated calves compared to saline-treated calves. Additionally, time to improvement was significantly less (P < 0.0001) in both studies for tulathromycin injection-treated calves compared to saline-treated calves.
Foot Rot-The effectiveness of tulathromycin injection for the treatment of bovine foot rot was evaluated in 170 cattle in two field studies. Cattle diagnosed with bovine foot rot were enrolled and treated with a single subcutaneous dose of tulathromycin injection (2.5 mg/kg BW) or an equivalent volume of saline. Cattle were clinically evaluated 7 days after treatment for treatment success, which was based on defined decreases in lesion, swelling, and lameness scores. In both studies, the treatment success percentage was statistically significantly higher in tulathromycin injection-treated calves compared with saline-treated calves (60% vs. 8%, P < 0.0001 and 83.3% vs. 50%, P = 0.0088).
Swine
In a multi-location field study to evaluate the treatment of naturally occurring SRD, 266 pigs were treated with tulathromycin injection. Responses to treatment were compared to saline-treated controls. Success was defined as a pig with normal attitude, normal respiration, and rectal temperature of < 104°F on Day 7. The treatment success rate was significantly greater (P ≤ 0.05) in tulathromycin injection-treated pigs (70.5%) compared to saline-treated pigs (46.1%). M. hyopneumoniae was isolated from 106 saline-treated and non-treated sentinel pigs in this study.
Two induced infection model studies were conducted to confirm the effectiveness of tulathromycin injection against M. hyopneumoniae. Ten days after inoculation intranasally and intratracheally with a field strain of M. hyopneumoniae, 144 pigs were treated with either tulathromycin injection (2.5 mg/kg BW) intramuscularly or an equivalent volume of saline. Pigs were euthanized and necropsied 10 days post-treatment. The mean percentage of gross pneumonic lung lesions was statistically significantly lower (P < 0.0001) for tulathromycin injection-treated pigs than for saline-treated pigs in both studies (8.52% vs. 23.62% and 11.31% vs. 26.42%).
The effectiveness of tulathromycin injection for the control of SRD was evaluated in a multi-location natural infection field study. When at least 15% of the study candidates showed clinical signs of SRD, all pigs were enrolled and treated with tulathromycin injection (226 pigs) or saline (227 pigs). Responses to treatment were evaluated on Day 7. Success was defined as a pig with normal attitude, normal respiration, and rectal temperature of < 104°F. The treatment success rate was significantly greater (P < 0.05) in tulathromycin injection-treated pigs compared to saline-treated pigs (59.2% vs. 41.2%).
Animal Safety
Cattle
Safety studies were conducted in feeder calves receiving a single subcutaneous dose of 25 mg/kg BW, or 3 weekly subcutaneous doses of 2.5, 7.5, or 12.5 mg/kg BW. In all groups, transient indications of pain after injection were seen, including head shaking and pawing at the ground. Injection site swelling, discoloration of the subcutaneous tissues at the injection site and corresponding histopathologic changes were seen in animals in all dosage groups. These lesions showed signs of resolving over time. No other drug-related lesions were observed macroscopically or microscopically.
An exploratory study was conducted in feeder calves receiving a single subcutaneous dose of 10, 12.5, or 15 mg/kg BW. Macroscopically, no lesions were observed. Microscopically, minimal to mild myocardial degeneration was seen in one of six calves administered 12.5 mg/kg BW and two of six calves administered 15 mg/kg BW.
A safety study was conducted in preruminant calves 13 to 27 days of age receiving 2.5 mg/kg BW or 7.5 mg/kg BW once subcutaneously. With the exception of minimal to mild injection site reactions, no drug-related clinical signs or other lesions were observed macroscopically or microscopically.
Swine
Safety studies were conducted in pigs receiving a single intramuscular dose of 25 mg/kg BW, or 3 weekly intramuscular doses of 2.5, 7.5, or 12.5 mg/kg BW. In all groups, transient indications of pain after injection were seen, including restlessness and excessive vocalization. Tremors occurred briefly in one animal receiving 7.5 mg/kg BW. Discoloration and edema of injection site tissues and corresponding histopathologic changes were seen in animals at all dosages and resolved over time. No other drug-related lesions were observed macroscopically or microscopically.
Storage Conditions
Store at 59° to 86°F (15° to 30°C). Exposure to temperature up to 104°F (40°C) may be tolerated provided the mean kinetic temperature does not exceed 77°F (25°C); however, such exposure should be minimized. Exposure to temperatures down to 36°F (2°C) may be tolerated. For 50 & 100 ml vials: Use within 60 days of the first puncture and puncture a maximum of 52 times. For 250, 500 & 1000 ml vials: Use within 60 days of the first puncture and puncture a maximum of 80 times. If using a needle or draw-off spike larger than 16 gauge discard any remaining product immediately after use.
How Supplied
Tulieve Injectable Solution is available in the following package sizes:
50 mL vial
100 mL vial
250 mL vial
500 mL vial
100 mL vial
Approved by FDA under ANADA # 200-723
Tulieve® is a registered trademark of Norbrook Laboratories Limited
Made in the UK
Manufactured by:
Norbrook Laboratories Limited, Newry, BT35 6PU, Co. Down, Northern Ireland
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SD5), contact Norbrook at 1-866-591-5777. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
Revised Feb 2022
022670I01
CPN: 1345028.0
9733 LOIRET BLVD., LENEXA, KS, 66219
| Telephone: | 913-599-5777 | |
| Fax: | 913-599-5766 | |
| Website: | www.norbrook.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
