The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Thyrosyn Tablets
This page contains information on Thyrosyn Tablets for veterinary use.The information provided typically includes the following:
- Thyrosyn Tablets Indications
- Warnings and cautions for Thyrosyn Tablets
- Direction and dosage information for Thyrosyn Tablets
Thyrosyn Tablets
This treatment applies to the following species: Manufacturer: Vedco
Manufacturer: Vedco
(LEVOTHYROXINE SODIUM) TABLETS
Thyrosyn Tablets Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description:
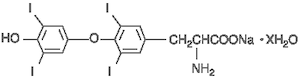
Each THYROSYN (Levothyroxine Sodium, USP) Tablet provides synthetic crystalline levothyroxine sodium (L-thyroxine).
The structural formula for levothyroxine sodium is:
Levothyroxine Sodium Action:
Levothyroxine sodium acts, as does endogenous thyroxine, to stimulate metabolism, growth, development and differentiation of tissues. It increases the rate of energy exchange and increases the maturation rate of the epiphyses. Levothyroxine sodium is absorbed rapidly from the gastrointestinal tract after oral administration. Following absorption, the compound becomes bound to the serum alpha globulin fraction. For purposes of comparison, 0.1 mg of levothyroxine sodium elicits a clinical response approximately equal to that produced by one grain (65 mg) of desiccated thyroid.
Thyrosyn Tablets Indications
Provides thyroid replacement therapy in all conditions of inadequate production of thyroid hormones. Hypothyroidism is the generalized metabolic disease resulting from deficiency of the thyroid hormones levothyroxine (T4) and liothyronine (T3). Thyrosyn (levothyroxine sodium) will provide levothyroxine (T4) as a substrate for the physiologic deiodination to liothyronine (T3). Administration of levothyroxine sodium alone will result in complete physiologic thyroid replacement.
Canine hypothyroidism is usually primary, i.e., due to atrophy of the thyroid gland. In the majority of cases the atrophy is associated with lymphocytic thyroiditis and in the remainder it is non-inflammatory and as of yet unknown etiology. Less than 10 percent of cases of hypothyroidism are secondary, i.e., due to deficiency of thyroid stimulating hormone (TSH). TSH deficiency may occur as a component of congenital hypopituitarism or as an acquired disorder in adult dogs, in which case it is invariably due to the growth of a pituitary tumor.
Hypothyroidism in the Dog:
Hypothyroidism usually occurs in middle-aged and older dogs although the condition will sometimes be seen in younger dogs of the larger breeds. Neutered animals of either sex are also frequently affected, regardless of age. The following are clinical signs of hypothyroidism in dogs:
Lethargy, lack of endurance, increased sleeping
Reduced interest, alertness and excitability
Slow heart rate, weak apex beat and pulse, low voltage on ECG
Preference for warmth, low body temperature, cool skin
Increased body weight
Stiff and slow movements, dragging of front feet
Head tilt, disturbed balance, unilateral facial paralysis
Atrophy of epidermis, thickening of dermis
Surface and follicular hyperkeratosis, pigmentation
Puffy face, blepharoptosis, tragic expression
Dry, coarse, sparse coat, slow regrowth after clipping
Retarded turnover of hair (carpet coat of boxers)
Shortening or absence of estrus, lack of libido
Dry feces, occasional diarrhea
Hypercholesterolemia
Normochromic, normocytic anemia
Elevated serum creatinine phosphokinase
Contraindications
Levothyroxine sodium therapy is contraindicated in thyrotoxicosis, acute myocardial infarction and uncorrected adrenal insufficiency. Use in pregnant bitches has not been evaluated.
Precautions
The effects of levothyroxine sodium therapy are slow in being manifested. Overdosage of any thyroid drug may produce the signs and symptoms of thyrotoxicosis including, but not limited to: polydipsia, polyuria, polyphagia, reduced heat tolerance and hyperactivity or personality change. Administer with caution to animals with clinically significant heart disease, hypertension or other complications for which a sharply increased metabolic rate might prove hazardous.
Adverse Reactions
There are no particular adverse reactions associated with levothyroxine sodium therapy at the recommended dosage levels. Overdosage will result in the signs of thyrotoxicosis listed above under precautions.
Dosage:
The initial recommended dose is 0.1 mg/10 lb. (4.5 kg) body weight twice daily. Dosage is then adjusted by monitoring the thyroid blood levels of the dog every four weeks until an adequate maintenance dose is established. The usual maintenance dose is 0.1 mg/10 lb. (4.5 kg) once daily.
DISPENSE IN THIS CONTAINER OR ANOTHER TIGHT, LIGHT-RESISTANT CONTAINERS AS DEFINED IN THE USP.
Administration:
Thyrosyn tablets may be administered orally or placed in the food.
Dosage forms available:
0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg, 0.5 mg, 0.6 mg, 0.7 mg, 0.8 mg and 1.0 mg tablets in bottles of 180 and 1,000.
Storage
Store at controlled room temperature 15°C to 30°C (59°F to 86°F).
References
1. Evinger, J.V. and Nelson, R.W.; JAVMA 314. 1984, pg 185, 314-316.
2. Richard Nelson, DVM; Current Veterinary Therapy X. Edited by R. W. Kirk, W.B. Saunders, Co., Philadelphia, PA 1989, pg 994.
3. Edward Feldman, DVM and Richard Nelson, DVM; Canine and Feline Endocrinology and Reproduction. W.B. Saunders 1987, pg 82.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Distributed By: VEDCO, INC., St. Joseph, MO 64507
For More Information Call 888-708-3326
08/12
302130-01
|
NET CONTENTS: |
|
NDC |
ID# |
|
|
180 TABLETS |
0.1 MG YELLOW TABLET |
50989-271-86 |
85111 |
302101-01 |
|
180 TABLETS |
0.2 MG PINK TABLET |
50989-272-86 |
85112 |
302102-01 |
|
180 TABLETS |
0.3 MG GREEN TABLET |
50989-273-86 |
85113 |
302103-01 |
|
180 TABLETS |
0.4 MG MAROON TABLET |
50989-274-86 |
85114 |
302104-01 |
|
180 TABLETS |
0.5 MG WHITE TABLET |
50989-275-86 |
85115 |
302105-01 |
|
180 TABLETS |
0.6 MG PURPLE TABLET |
50989-276-86 |
85116 |
302106-01 |
|
180 TABLETS |
0.7 MG ORANGE TABLET |
50989-267-86 |
85117 |
302107-01 |
|
180 TABLETS |
0.8 MG BLUE TABLET |
50989-278-86 |
85118 |
302108-01 |
|
180 TABLETS |
1.0 MG BEIGE TABLET |
50989-270-86 |
85110 |
302100-01 |
|
1000 TABLETS |
0.1 MG YELLOW TABLET |
50989-271-53 |
85121 |
302110-01 |
|
1000 TABLETS |
0.2 MG PINK TABLET |
50989-272-53 |
85122 |
302111-01 |
|
1000 TABLETS |
0.3 MG GREEN TABLET |
50989-273-53 |
85123 |
302112-01 |
|
1000 TABLETS |
0.4 MG MAROON TABLET |
50989-274-53 |
85124 |
302113-01 |
|
1000 TABLETS |
0.5 MG WHITE TABLET |
50989-275-53 |
85125 |
302114-01 |
|
1000 TABLETS |
0.6 MG PURPLE TABLET |
50989-276-53 |
85126 |
302115-01 |
|
1000 TABLETS |
0.7 MG ORANGE TABLET |
50989-267-53 |
85127 |
302116-01 |
|
1000 TABLETS |
0.8 MG BLUE TABLET |
50989-278-53 |
85128 |
302117-01 |
|
1000 TABLETS |
1.0 MG BEIGE TABLET |
50989-270-53 |
85120 |
302109-01 |
NAC No.: 1094199.2
5503 CORPORATE DR., ST. JOSEPH, MO, 64507
| Telephone: | 816-238-8840 | |
| Toll-Free: | 888-708-3326 (888-70VEDCO) | |
| Fax: | 816-238-1837 | |
| Website: | www.vedco.com |
 |
Every effort has been made to ensure the accuracy of the Thyrosyn Tablets information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
