Senvelgo (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
Velagliflozin Oral Solution 15 mg/mL
Veterinary Use Only
For cats
DIN 02543427
Description
Senvelgo™ (velagliflozin oral solution) is a clear, colourless to slightly yellow, to slightly brown, multi-dose preparation consisting of 15 mg/mL velagliflozin intended for oral use in cats. Senvelgo is a sodium-glucose co-transporter 2 (SGLT-2) inhibitor.
Non-medicinal ingredients: ethanol (96%), propylene glycol, citric acid monohydrate, sodium hydroxide, honey flavour, and purified water.
Senvelgo Indications
For the reduction of hyperglycemia in cats with non-insulin dependent diabetes mellitus.
Active Ingredient
Each mL contains 15 mg velagliflozin (as velagliflozin L-proline monohydrate).
Directions for use: Read entire package insert before use.
The recommended dosing regimen is once daily orally, 1 mg/kg body weight.
The solution should be drawn using the dosing syringe provided in the carton. The syringe fits onto the bottle and has a kilogram body weight scale in 0.5 kg increments. The product may be administered either directly into the mouth or with a small amount of food. The medication should be given at approximately the same time every day.
If a dose is missed, it should be given as soon as possible on the same day.
After administration close bottle tightly with the cap. The syringe can be cleaned with a clean, dry cloth.
Prior to starting treatment:
Ensure the cat is alert, active, eating and drinking and a complete work up for diabetes mellitus (exam, body weight, complete blood count, serum chemistry, fructosamine, Spec fPL, TT4, IGF-1, urinalysis, urine culture) and concurrent conditions has been done immediately before starting treatment, including evaluating for ketonuria (see Contraindications).
Monitoring of cats receiving Senvelgo:
Monitor the cat for hyporexia/anorexia, lethargy, dehydration, illness and weight loss. Immediately discontinue Senvelgo should any of those signs occur and assess the cat for diabetic ketoacidosis, no matter the blood glucose concentration.
During the first 2 weeks after starting treatment: evaluate the cat for ketonuria every 1 to 3 days and any time the cat shows signs of illness. If ketonuria is present, discontinue Senvelgo and treat with insulin (see below).
Two to three days after starting treatment: examine, weigh and measure blood glucose and assess a urine dip stick, especially for ketones.
One week and 1 month after starting treatment: examine, weigh, and do a complete blood count, serum chemistry, blood glucose curve, urinalysis, and serum fructosamine (1 month) to assess the cat. If there is poor glycemic control after 4 weeks of treatment or continued unintended weight loss, Senvelgo should be discontinued and insulin therapy initiated.
Thereafter the owner should assess the cat every 30 days. If there are changes to the cat’s condition, immediately reassess. Senvelgo should be discontinued if the cat’s condition deteriorates and/or glycemic control worsens after initial improvement.
Diabetic ketoacidosis or euglycemic ketoacidosis requires the following actions:
● Discontinue Senvelgo
● Initiate insulin therapy immediately, regardless of blood glucose concentration
● Administer dextrose or other carbohydrate source, if the cat has euglycemic ketoacidosis
● Provide appropriate nutritional support to prevent or treat hepatic lipidosis
Contraindications
Senvelgo is contraindicated in cats
● with insulin dependent diabetes mellitus,
● previously treated with insulin or receiving insulin (see DKA Adverse Events),
● with ketonuria
● with evidence of diabetic ketoacidosis, or
● severe dehydration requiring intravenous fluid supplementation.
Do not initiate treatment or continue treatment in cats with anorexia, dehydration, lethargy, clinical pancreatitis, or any condition or treatment that can cause insulin resistance.
Do not use in cases of hypersensitivity to the active substance or to any of the excipients.
Cautions:
Based on the mode of action of SGLT-2 inhibitors (such as velagliflozin), adequate endogenous insulin production is a requirement for successful management of diabetes mellitus with this veterinary medicinal product.
Cats treated with Senvelgo may be at an increased risk of diabetic ketoacidosis or euglycemic ketoacidosis. As diabetic ketoacidosis and euglycemic ketoacidosis in cats treated with Senvelgo may result in death, the ketoacidosis must be immediately treated including insulin administration and discontinuation of Senvelgo (see Monitoring).
Due to the mode of action of SGLT-2 inhibitors, high blood glucose levels will not be present in cases of DKA (euglycemic ketoacidosis). Therefore, the diagnosis of euglycemic DKA needs to be based on the presence of urine ketone bodies, and/or clinical signs.
Presence of acetoacetate and acetone ketone bodies can be checked by cat owners at home by dipping a urine test strip into the cat’s urine. Ketone body beta-hydroxybutyric acid (BHA) can be measured in the blood. Not all three ketones may be present in a cat with DKA.
The safety and efficacy of a combined treatment with insulin or other blood glucose lowering treatments and velagliflozin in cats has not been investigated. Due to the mode of action of insulin there is an increased risk for hypoglycemia, therefore combined treatment is not recommended.
Due to the mode of action, SGLT-2 inhibitors may cause an increase in serum creatinine, blood urea nitrogen (BUN), phosphorus, and sodium within weeks of starting therapy, followed by a stabilization of values. Routine evaluation of renal function, body weight and hydration status in patients with renal disease is recommended.
Use of velagliflozin in cats with chronic renal insufficiency has not been fully investigated.
Initial mild weight loss may be seen with Senvelgo associated with its mode of action (glucosuria and caloric wasting). If weight loss doesn’t improve or stabilize within 7 days consider evaluating for concurrent disease and discontinuing Senvelgo.
Senvelgo administration is associated with an increased risk of developing a urinary tract infection due to the persistent glucosuria. Routine urine culture is recommended.
Remission of diabetes mellitus in cats treated with Senvelgo has not been evaluated.
Glucosuria is not a reliable indicator for monitoring diabetic control while a cat is receiving a SGLT-2 inhibitor or shortly after discontinuation. Glucosuria may persist for 2-3 days after stopping Senvelgo.
Senvelgo contains propylene glycol. When cats are administered Senvelgo at the 1 mg/kg/day dose, cats receive 40 mg/kg/day of propylene glycol. Propylene glycol above 80 mg/kg/day can cause Heinz bodies to form without anemia. Use caution when administering Senvelgo to cats receiving other products that contain propylene glycol.
Use of Senvelgo following previous treatment with other oral anti-diabetic drugs has not been evaluated.
The safety of this product has not been established during breeding, pregnancy and lactation.
Warnings: Keep out of reach of children. Caution should be taken to avoid accidental ingestion and contact with eyes. This product can cause eye irritation; in case of contact with eyes immediately rinse thoroughly with water. In case of ingestion or contact with the eyes seek medical advice.
Adverse Reactions
A total of 408 patients from the USA, EU and Japan with diabetes mellitus were included in clinical trials to evaluate the safety and efficacy of velagliflozin, of which 343 patients received velagliflozin. Many adverse reactions observed were expected, based on the pharmacodynamic effect of SGLT-2 inhibitors. The osmotic diuresis accompanying glucosuria may lead to polyuria and/or polydipsia and weight loss. Minimal inhibition of SGLT-1 located in the intestine may lead to a softening of stool.Adverse Reactions Observed in 3 Clinical Field Studies (USA, EU and Japan) in 343 cats
|
Adverse Reactions |
Percent of Cats (n=343) |
|
Diarrhea/Loose stool |
51% |
|
Vomiting |
41% |
|
Hypersalivation |
38% |
|
Weight Loss |
37% |
|
Anorexia/Decreased Appetite |
21% |
|
Lethargy/Malaise |
20% |
|
Dehydration/dry mucous membranes |
14% |
|
Polydipsia |
14% |
|
Diabetic ketoacidosis/ketonuria/metabolic ketosis |
11% |
|
Renal Insufficiency1 |
11% |
1 This term includes cases with azotemia, chronic renal failure, increased BUN, increased SDMA, increased creatinine, increased protein:creatinine ratio, increased renal parameters, renal failure and renal insufficiency.
Electrolytes changes that are observed with velagliflozin are hypercalcemia, and hyperphosphatemia.
Velagliflozin may cause transient hypoglycemia but no adverse effects were observed.
In three clinical field efficacy studies, serious adverse events observed were diabetic ketoacidosis, euglycemic ketoacidosis, chronic renal insufficiency, dehydration and anorexia.
Diabetic Ketoacidosis
In the EU field study there were 4 cats (6.6%) treated with velagliflozin that developed euglycemic ketoacidosis. In 3 of the cats the euglycemic ketoacidosis occurred within the first week of treatment. In the fourth cat it occurred on study day 80. In the US field study 33 cats (13.1%) had diabetic ketoacidosis, diabetic ketonuria or ketosis. Most of the cats developed ketonuria before study day 7.
The risk for ketoacidosis is greatest within the first 14 days of starting treatment. Eighty-four percent of cats that developed ketoacidosis did so within the first 14 days of treatment. The incidence of clinical DKA within the first two weeks after starting treatment with velagliflozin was 5.1% in insulin naïve cats and 12.1% in cats pre-treated with insulin.
In the EU field study 13 (21.3%) velagliflozin treated cats and 10 (15.2%) insulin treated cats were treated for cystitis.
Death and Euthanasia
In the EU field study, 4 (6.6%) velagliflozin treated cats and 5 (7.6%) insulin treated cats died or were euthanized. In the velagliflozin group 1 cat died of advanced renal failure, one cat had yellow mucous membranes, decreased appetite, decreased weight and diarrhea, the third cat had decreased appetite, polydipsia, diarrhea and the fourth cat was dehydrated, lethargic and recumbent.
In the US field study, 17/252 (6.7%) cats died or were euthanized during the study or shortly after their removal from the study. The cats had acute renal failure (1), diabetic ketoacidosis (3), pancreatitis and hyperlipidemia (3), urinary incontinence/inappropriate urination (3), progressive diabetes mellitus (1), neoplasia (3), heart failure (1), pneumonia (1) or behaviour change-aggressive (1).
Clinical Pharmacology
Key Scientific Information: Velagliflozin as velagliflozin L-proline monohydrate is a sodium-glucose co-transporter 2 (SGLT-2) inhibitor. The chemical name is 2 - (4 - cyclopropylbenzyl) - 4 - ((2S,3R,4R,5S,6R) - 3,4,5 - trihydroxy - 6 - hydroxymethyltetrahydropyran - 2 - yl) - benzonitrile (S)-pyrrolidine-2-carboxylic acid monohydrate.
The chemical structure is:
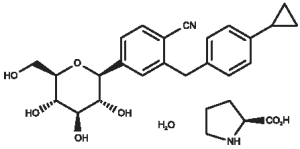
Pharmacodynamics: Velagliflozin inhibits the sodium-glucose co-transporter 2 (SGLT-2) which is predominantly expressed in the kidney. SGLT-2 is the primary transporter for the reabsorption of glucose from the urine. The inhibition of the SGLT-2 leads to glucose elimination in the urine and consequently results in a decrease in elevated blood glucose levels in diabetic cats. Velagliflozin treatment leads to a decrease in serum blood glucose values below the renal threshold of 16.7mmol/L throughout the day. This decrease is usually observed within 7 days after start of treatment.
Increased glucose levels in the urine may lead to an increase in osmotic diuresis (increased urine volume). Once blood glucose levels in diabetic cats are balanced and the excretion of glucose is decreased, the clinical signs of polyuria and polydipsia improve.
Polyphagia (increased appetite) may not improve instantly or completely, due to the mode of action of SGLT-2 inhibitors, inducing glucosuria and therefore energy loss, which may lead to compensatory food uptake to prevent unintended weight loss.
Although velagliflozin is selective for SGLT-2, a potential minor effect on SGLT-1, present in the intestine, may lead to a dose dependent softening of stool and cause diarrhea/loose stool (mostly transient).
Pharmacokinetics:
After oral administration of velagliflozin to fasted cats, plasma-concentration-time curves were used to generate the following pharmacokinetic parameters:
|
PK parameter |
Fed |
Fasted |
|
Tmax (h) |
1.00 - 3.67 |
0.58 - 1.00 |
|
Cmax (ng/mL) |
315.7 - 846.0 |
1293.3 - 2160.7 |
|
AUC h*ng/mL |
2785.7 - 7141.7 |
6944.3 - 11035.0 |
|
T1/2 (h) |
4.71 - 6.44 |
4.49 - 5.51 |
In summary fasted cats showed a higher Cmax and shorter Tmax, resulting in a higher exposure (AUC0-24h) compared to cats in fed state. After repeat daily oral administration of 1, 3 and 5 mg/kg velagliflozin to cats over six months, a slight increase of exposure (range: 1.3 to 1.9-fold) was observed. In addition, a tendency for a less than dose-proportional increase of exposure (AUC) and Cmax was observed for all dose levels.
No relevant difference in exposure was observed between male and female cats.
Distribution:
An in vitro study using cat plasma showed high binding of velagliflozin to plasma proteins (91.3-93.7%). An in vitro study using cat whole blood showed moderate partitioning of velagliflozin into red blood cells. Blood cell concentration versus plasma concentration ratio (Cbc/Cp) was 0.84-0.88. Pharmacokinetics after intravenous administration to cats showed a medium volume of distribution (Vss) of 0.63 L/kg.
Metabolism:
Velagliflozin showed an absolute bioavailability of 96% in healthy fasted cats after oral administration. Metabolic pathways observed in cats after oral administration of velagliflozin include oxidation, a combination of oxidation and dehydrogenation, and sulfate conjugation, a minor metabolic pathway.
Elimination:
After oral administration (fed/fasted) mean half-life (T1/2) ranged from 4.49 to 6.44 hours. After oral administration to cats, 54% of velagliflozin was primarily excreted in the feces of which 72% was the parent drug. Only minor renal excretion occurred (approx. 4%).
Animal Safety
A 6-month placebo-controlled study was conducted to evaluate the safety of velagliflozin 15 mg/mL oral solution when administered once daily for 6 months to healthy 8-9 month old male and female cats, (4 male and 4 female cats per group) at 0x, 1x, 3x, and 5x the maximum daily target dose of 1 mg/kg body weight.
All animals survived the study and there was no evidence of velagliflozin effects on rectal temperature, respiratory and heart rates, ophthalmoscopy findings, systolic blood pressure, blood coagulation parameters as well as organ weights at necropsy could be identified. No histological alterations were observed. Hypersalivation and vomiting occurred infrequently after velagliflozin administration.
Loose feces, increased water consumption, increased food consumption and decreased weight gain (growing cats) were findings clearly attributable to the treatment.
There were drug-related increases in reticulocytes count, mean corpuscular hemoglobin, mean corpuscular volume, and Heinz bodies percentages in cats treated with velagliflozin compared to placebo treated cats. None of the cats showed clinical signs of anemia and the mean number of erythrocytes, hemoglobin and hematocrit values were within the normal range; however one 1x, one 3x and one 5x cat had red blood cell counts below the normal range. Velagliflozin did not affect white blood cells and platelets counts.
Treatment related increases in serum triglyceride, cholesterol, magnesium, calcium and albumin values were observed in cats that received velagliflozin. For all these parameters mean values stayed within the reference range; however, some magnesium, serum albumin and triglyceride values were above the reference range. There was a decrease in the mean BUN values in all velagliflozin treated groups. No other treatment effects were observed including serum glucose and symmetric dimethylarginine (SDMA). Glucosuria occurred in all the treatment groups corresponding to the pharmacodynamic effect of velagliflozin which causes glucose excretion through the kidney.
A reticular pattern was observed on the surface of the liver of one control, three 1x, four 3x and three 5x group cats.
Efficacy
In a European clinical field trial, the efficacy of 1 mg/kg once daily oral velagliflozin in 61 client owned diabetic cats was evaluated and compared to twice daily veterinary licensed porcine insulin therapy (individual dose adjustment) in 66 client owned diabetic cats over 91 days. The mean age of the cats was 11.0 +/- 2.9 years and the mean weight was 4.9 kg. The cats were evaluated at Days 3, 7, 21, 45 and 90. Treatment success was evaluated on Day 45 and was defined as an improvement in at least one clinical parameter (water consumption, frequency or volume of urination, appetite or diabetic neuropathy) and improvement in one blood glucose parameter (mean blood glucose of the blood glucose curve ≤14 mmol/L, minimum blood glucose ≤9 mmol/L on a 9 hour blood glucose curve or serum fructosamine <450 μmol/L). Of the 54 velagliflozin cats evaluated for efficacy 29 (53.7%) were considered treatment successes compared to 26 of 62 (41.9%) insulin treated cats. Treatment success on Day 91 was 30/54 (55.6%) in velagliflozin treated cats and 39/62 (62.9%) in insulin treated cats.
Two hundred and fifty-two (252) cats diagnosed with diabetes mellitus were enrolled in a 180-day multicentre US field study. The cats were 4 to 18 years of age and weighed 2.6-12 kg. Cats were administered Senvelgo at dose of 1 mg/kg orally, once daily, regardless of the glucose concentration, beginning on Day 0. The cats were evaluated at Days 2 or 3, and days 7 and 30 and then monthly.
Treatment success was evaluated on Day 30 and was defined as an improvement in at least one clinical sign of diabetes mellitus (polyuria, polydipsia, unintended weight loss, polyphagia, or diabetic neuropathy) and improvement in at least one blood glucose variable (blood glucose curve mean <16.7 mmol/L or serum fructosamine <450 μmol/l). Of the 198 cats included in the efficacy population 175 cats (88.4%) were considered a treatment success on Day 30. One hundred and fifty-seven cats completed the 180 day study.
Storage
Store between 15 - 30°C. Upon opening, use within 6 months.Presentation: Senvelgo is supplied in a polyethylene bottle containing 30 mL with a polyethylene plug-in and a child resistant closure. Each bottle is packaged in a carton that includes a dosing syringe.
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
Senvelgo is a trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
Date of Last Revision: 11-2023
L6508
CPN: 1182206.0
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
