Rheumocam 1.5 mg/ml oral suspension for dogs (Canada)
This treatment applies to the following species: Company: Merck Animal Health
Company: Merck Animal Health
Veterinary Use Only
DIN:02389932
Description
Each ml contains 1.5 mg of meloxicam in a yellowish suspension with an odour of honey. Meloxicam acts by inhibition of prostaglandin synthesis.
Therapeutic classification: Rheumocam 1.5 mg/ml oral suspension for dogs is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam group.
Rheumocam 1.5 mg/ml oral suspension for dogs Indications
Rheumocam 1.5 mg/ml oral suspension for dogs is indicated in dogs only, for the alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.
Dosage and Administration
Rheumocam 1.5 mg/ml oral suspension for dogs should be administered mixed with food. On the first day of treatment, a single dose of 0.2 mg meloxicam/kg body weight should be given. Treatment is to be continued once daily by oral administration (at 24-hour intervals) at a maintenance dose of 0.1 mg meloxicam/kg body weight.Particular care should be given with regard to the accuracy of dosing. The suspension can be given using the measuring syringe provided in the package (see below). The syringe has a kg-body weight scale which corresponds to the volume required for the maintenance dose (i.e., 0.1 mg Meloxicam per kg body weight). Twice the volume should be administered on the first day as the initial dose.
The following dosing table indicates what volume to administer depending on the weight of the dog:
|
Bodyweight |
Maintenance Dosage |
|
7.5 kg |
0.5 ml |
|
15 kg |
1 ml |
|
22.5 kg |
1.5 ml |
|
30 kg |
2 ml |
|
37.5 kg |
2.5 ml |
|
45 kg |
3 ml |
|
52.5 kg |
3.5 ml |
|
60 kg |
4 ml |
Shake well before use. Please follow carefully the instructions of the veterinarian.
Dosing Procedure using the measuring syringe:
Please follow these steps:
Step 1.
Before using Rheumocam 1.5 mg/ml oral suspension for dogs for the very first time ensure that you have the bottle, circular plastic insert and syringe.
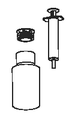
Step 2.
Place the circular plastic insert into the neck of the bottle and push down until securely in place. Once in place the insert will not need to be removed.
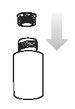
Step 3.
Replace the cap onto the bottle and shake it well. Take off the bottle cap and attach the dosing syringe to the bottle by gently pushing the end into the hole.
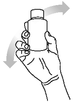
Step 4.
Turn the bottle with the syringe in place upside down and slowly withdraw the plunger until the required dose is evident.
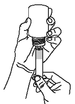
Step 5.
Turn the bottle/syringe the right way up and with a twisting movement separate the syringe from the bottle.
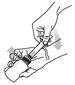
Step 6.
Push the plunger until all contents of the syringe have been dispensed onto the food.
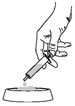
A clinical response is normally seen within 3 to 4 days. Treatment should be discontinued after 10 days at the latest if no clinical improvement is apparent.
Contraindications
Rheumocam 1.5 mg/ml oral suspension for dogs should not be administered if gastric or intestinal ulceration or bleeding is suspected; if there is evidence of cardiac, hepatic or renal disease; or if there is evidence of a haemorrhagic disorder or individual hypersensitivity to the product. Do not administer concurrently, other steroidal or nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics or anti-coagulant agents. Pre-treatment with other steroidal or nonsteroidal anti-flammatory drugs (NSAIDS) may result in additional or increased effects. Depending on the pharmacokinetic properties of the products used previously, an appropriate treatment-free period with such drugs should be observed before commencement of treatment.
Rheumocam 1.5 mg/ml oral suspension for dogs Caution
Not approved for use in cats.
Rheumocam 1.5 mg/ml oral suspension for dogs should not be administered to breeding, pregnant or lactating dogs. Do not exceed the stated dose. In case of overdosing, symptomatic treatment should be initiated.
Animals being treated with meloxicam should be monitored for the occurrence of side effects as susceptibility varies with the individual.
If gastrointestinal or other adverse reactions occur, treatment should be discontinued.
As for all NSAIDs, use in debilitated aged animals may involve additional risk. If use in such animals cannot be avoided, a reduced dosage and careful clinical management may be required. Safety of meloxicam in very young puppies has not been evaluated.
Warnings: Keep Out Of Reach Of Children.
Adverse Reactions
Typical adverse reactions of NSAIDs such as loss of appetite, vomiting, diarrhea, apathy and renal failure have occasionally been reported. These adverse reactions are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal.
Although all adverse reactions are not reported, the following adverse reaction information is based on voluntary reporting by veterinarians and pet owners/caregivers. It should be noted that suspected adverse reactions listed here reflect reporting, not causality. The categories of adverse reactions are listed in decreasing order of frequency by body system. In rare cases, death has been associated with some of these adverse reactions.
Digestive tract: vomiting, diarrhea, melena, hematemesis, gastric ulcer
Systemic: anorexia, lethargy, jaundice
Renal and urinary: elevated creatinine, elevated BUN, acute renal failure
Neurological: ataxia, seizure, sleepiness, trembling
Behavioural: behavioural disorder, hyperactivity
Skin and appendages: pruritus, eczema, alopecia local, moist dermatitis
Immune system: urticaria, allergic dermatitis, immune mediated hemolytic anemia, immune mediated thrombocytopenia
Hepato-biliary: Elevated liver enzymes
Information for Pet Owners: Rheumocam 1.5 mg/ml oral suspension for dogs (Meloxicam) is a nonsteroidal anti-inflammatory drug (NSAID) and as with others in this class adverse reactions may occur in treated dogs. The most common adverse reactions reported involve the gastrointestinal tract and usually occur within the first week of treatment. Typical symptoms include loss of appetite, vomiting, diarrhea, dark stools and depression. It is important in these situations to discontinue treatment and contact your veterinarian. In most cases, the adverse reactions are transient and disappear after termination of treatment but in rare instances may be serious or fatal especially if treatment is not discontinued. Dogs undergoing prolonged treatment with Rheumocam 1.5 mg/ml oral suspension for dogs should be monitored periodically. Consult your veterinarian. Shake well before use.
Safety: The safety profile of meloxicam has been evaluated in well controlled target animal safety studies in dogs. Dogs treated with placebo, 1X, 3X and 5X label dosages were closely monitored over a 180 day (26 week period). The study determined that there were no drug related adverse reactions on clinical observations, normal body weight gain, food consumption, physical and ophthalmic examinations, clotting times, mucosal bleeding times or on a panel of clinical pathology parameters monitored throughout the study.
Storage
Store between 15-30° C.Presentation: Plastic bottles of 15, 42, 100 or 200 ml supplied with a measuring syringe.
Manufactured by: Chanelle Pharmaceuticals Manufacturing Ltd., Loughrea, Co. Galway, Ireland
Distributed by: Intervet Canada Corp. subsidiary of Merck & Co., Inc., 16750 Trans-Canada Highway, Kirkland, Quebec H9H 4M7
1-888-306-0069
Date: 26th July 2012
LA7689
CPN: 12082580
Intervet Canada Corp.
16750 ROUTE TRANSCANADIENNE, KIRKLAND, QC, H9H 4M7
| Order Desk: | 514-428-7013 | |
| Toll-Free: | 866-683-7838 | |
| Fax: | Toll-free 888-498-4444; local 514-428-7014 | |
| Website: | www.merck-animal-health.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
