PANOQUELL-CA1 (fuzapladib sodium for injection)
This treatment applies to the following species: Company: Ceva Animal Health
Company: Ceva Animal Health
4 mg/mL when reconstituted
Leukocyte function-associated antigen 1 (LFA-1) activation inhibitor
For intravenous use in dogs only
PANOQUELL-CA1 (fuzapladib sodium for injection) Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-567.
It is a violation of Federal law to use this product other than as directed in the labeling.
Description
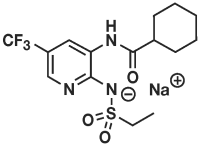
PANOQUELL®-CA1 (fuzapladib sodium for injection) is a selective inhibitor of LFA-1. Fuzapladib sodium is the non-proprietary designation for N-[2-((ethylsulfonyl)amino)-5-(trifluoromethyl)-3-pyridinyl] cyclohexanecarboxamide monosodium. PANOQUELL®-CA1 has a molecular weight of 401.38 and the following structural formula:
PANOQUELL®-CA1 consists of two separate vials. One vial contains 14 mg of fuzapladib sodium, 52.5 mg of D-mannitol, and 21 mg of tromethamine as sterile lyophilized powder. The second vial of 3.9 mL sterile diluent (bacteriostatic water for injection), containing 1.8% w/v benzyl alcohol, is for reconstituting the sterile lyophilized powder prior to use. No other diluent should be used. When reconstituted with 3.5 mL of the provided Bacteriostatic Water for Injection, each milliliter of reconstituted drug product contains 4 mg fuzapladib sodium, 15 mg D-mannitol, 6 mg tromethamine, and 18 mg benzyl alcohol. The pH was adjusted with hydrochloric acid or sodium hydroxide.
INDICATION:
PANOQUELL®-CA1 is indicated for the management of clinical signs associated with acute onset of pancreatitis in dogs.
Dosage and Administration
Prior to use, the sterile lyophilized powder should be reconstituted (see Reconstitution Procedures) using the sterile diluent provided, resulting in a 4 mg/mL solution of PANOQUELL®-CA1. Once reconstituted, swirl the bottle gently before every use to ensure a uniform solution.
The reconstituted product is administered at a dosage of 0.4 mg (0.1 mL) per kg body weight once daily for three consecutive days by intravenous (IV) bolus injection over 15 seconds to 1 minute.
Reconstitution Procedures:
The items needed for reconstitution are:
● Sterile PANOQUELL®-CA1 lyophilized powder
● Sterile diluent
● Sterile 5 mL syringe for transfer of diluent
● Sterile needle
Steps for reconstitution:
1. Using a sterile needle and syringe, withdraw 3.5 mL of the sterile diluent from the vial and slowly transfer the sterile diluent into the vial containing the sterile PANOQUELL®-CA1 lyophilized powder through the stopper. There is more sterile diluent supplied than the 3.5 mL needed for reconstitution.
2. Once the sterile diluent has been added to the powder vial, remove the needle and syringe from the vial. Discard unused sterile diluent, syringe, and needle.
3. Gently swirl the vial until the powder is fully reconstituted into solution, leaving no visible residue or undissolved material.
4. Before each use, gently swirl to ensure a uniform solution.
5. Draw up the appropriate dose using a new sterile needle and syringe.
6. Administer the dose promptly after drawing into the dosing syringe.
7. Store any remaining reconstituted product at refrigerated conditions, 36° to 46°F (2° to 8°C). Reconstituted product remains stable under refrigeration for 28 days.
Contraindications
Do not use in dogs with a known hypersensitivity to fuzapladib sodium.
Warnings
User Safety Warnings:
Not for use in humans. Keep this medication out of reach of children.
In case of accidental self-injection:
● Seek medical advice immediately and show the package insert or label to the physician.
In case of skin contact:
● Wash the exposed skin with water for at least 15 minutes.
● If redness and swelling occur, seek medical advice immediately and show the package insert or label to the physician.
In case of accidental eye exposure:
● Wash the eyes with water for at least 15 minutes.
● If wearing contact lenses, rinse the eyes first, then remove contacts and continue to rinse with water.
● If redness and swelling occur, seek medical advice immediately and show the package insert or label to the physician.
In case of accidental ingestion:
● Rinse the mouth out with water.
● Do not induce vomiting unless directed to do so by medical personnel.
● Seek medical advice immediately and show the package insert or label to the physician.
Limited data is available on the potential teratogenic effects of fuzapladib sodium. Therefore, anyone who is pregnant, breast feeding, or planning to become pregnant should avoid direct contact with PANOQUELL®-CA1.
Anyone with known hypersensitivity to fuzapladib sodium or to any of the excipients should avoid contact with PANOQUELL®-CA1.
To obtain a Safety Data Sheet, contact Ceva Animal Health, LLC, at 1-800-999-0297 or www.ceva.com.
Precautions
PANOQUELL®-CA1 is highly protein bound. Use with caution with other medications that are highly protein bound. The concomitant use of PANOQUELL®-CA1 with other protein bound drugs has not been studied in dogs. Commonly used protein bound drugs include non-steroidal anti-inflammatory drugs (NSAIDs), anti-emetics, antibiotics, diuretics, and behavioral medications. Drug compatibility should be monitored in patients requiring adjunctive therapy. Concurrent medications used during the pilot effectiveness study with fuzapladib sodium included, but were not limited to, pain medications (excluding NSAIDs), anti-emetics, parasiticides, vaccinations, and medications used to treat well-controlled pre-existing conditions.
The safe use of PANOQUELL®-CA1 has not been evaluated in dogs with cardiac disease, hepatic failure, or renal impairment.
The safe use of PANOQUELL®-CA1 has not been evaluated in dogs that are pregnant, lactating, or intended for breeding.
The safe use of PANOQUELL®-CA1 has not been evaluated in dogs less than 6 months of age.
Adverse Reactions
In a well-controlled pilot field study to assess the effectiveness and safety of fuzapladib sodium (not commercial formulation) in client-owned dogs diagnosed with acute onset of pancreatitis (see REASONABLE EXPECTATION OF EFFECTIVENESS), 31 dogs administered fuzapladib sodium and 30 dogs administered vehicle control were evaluated for safety. The vehicle control was excipient sterile lyophilized powder solubilized in 1 mL of Sterile Water for Injection, USP. The adverse reactions observed in the study and the number of dogs experiencing each adverse reaction is summarized in Table 1.
Table 1: Adverse Reactions During the Pilot Field Study
|
Adverse Reaction |
Fuzapladib Sodium (n = 31) (%) |
Vehicle Control (n = 30) (%) |
|
Anorexia |
5 (16.1%) |
2 (6.7%) |
|
Digestive tract disorders |
5 (16.1%) |
3 (10.0%) |
|
Respiratory tract disorders |
4 (12.9%) |
3 (10.0%) |
|
Hepatopathy, jaundice |
4 (12.9%) |
2 (6.7%) |
|
Abnormal urine |
3 (9.7%) |
2 (6.7%) |
|
Diarrhea |
3 (9.7%) |
1 (3.3%) |
|
Arrhythmia |
2 (6.5%) |
1 (3.3%) |
|
Cardiac arrest |
2 (6.5%) |
0 |
|
Hyperthermia |
2 (6.5%) |
0 |
|
Pruritis, urticaria |
2 (6.5%) |
0 |
|
Hypersalivation |
2 (6.5%) |
0 |
|
Heart murmur |
1 (3.2%) |
2 (6.7%) |
|
Limb edema |
1 (3.2%) |
2 (6.7%) |
|
Subcutaneous swelling, bruising at injection site |
1 (3.2%) |
1 (3.3%) |
|
Tremor/shivering/shaking |
1 (3.2%) |
1 (3.3%) |
|
Abrasion |
1 (3.2%) |
1 (3.3%) |
|
Cerebral edema |
1 (3.2%) |
0 |
|
Anaphylaxis |
1 (3.2%) |
0 |
|
Hypertension |
1 (3.2%) |
0 |
In Table 1, digestive tract disorders included regurgitation (1 fuzapladib, 2 vehicle control), vomiting (1 fuzapladib, 1 vehicle control), flatulence (1 fuzapladib), nausea (1 fuzapladib), and enteritis (1 fuzapladib). Respiratory tract disorders included pneumonia (2 fuzapladib, 1 vehicle control), inspiratory crackles (1 fuzapladib, 2 vehicle control), tachypnea (2 fuzapladib), and dyspnea (1 fuzapladib). Abnormal urine included proteinuria (2 fuzapladib, 2 vehicle control), hematuria (2 vehicle control), and malodorous urine (1 fuzapladib). Some of these dogs were reported with more than one abnormality.
Note: Some dogs experienced an adverse reaction on more than one occasion but are only presented once in the table above for each reported adverse reaction.
Five out of the 61 enrolled dogs died during the study: four in the fuzapladib sodium group and one in the vehicle control group. Two additional dogs in the vehicle control group were euthanized shortly after completion of the study. Of the seven dogs that died or were euthanized during or after the study, three deaths could be attributed to complications from severe acute onset of pancreatitis: two in the fuzapladib sodium group and one in the vehicle control group. One dog in the fuzapladib sodium group was suspected to have aspiration pneumonia and died after experiencing cardiac arrest. One dog in the vehicle control group was euthanized because of a poor prognosis. Two deaths could be attributed to causes other than acute onset of pancreatitis: one dog in the fuzapladib sodium group had intestinal lymphoma and one vehicle control group dog had a cranial thromboembolic event and a pheochromocytoma.
Foreign Market Experience
The following adverse events were reported voluntarily during post-approval use of the product in dogs in foreign markets: facial and tongue swelling, collapse, and seizure. These adverse events occurred within 24 hours of administration.
CONTACT INFORMATION:
Contact Ceva Animal Health, LLC, at 1-800-999-0297 or www.ceva.com. To report suspected adverse drug experiences or for technical assistance, contact Ceva Animal Health, LLC, at 1-800-999-0297.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or at www.fda.gov/reportanimalae
Clinical Pharmacology
Mechanism Of Action
PANOQUELL®-CA1 has anti-inflammatory effects through its ability to inhibit activation of LFA-1, resulting in inhibition of inflammatory cell adhesion and migration into sites of tissue injury and inflammation. These anti-inflammatory properties are thought to limit pancreatic lesion expansion and help prevent complications such as multi-organ failure.
Pharmacokinetics
Following once daily IV administration of PANOQUELL®-CA1 at 0.4 mg/kg, 1.2 mg/kg, and 2 mg/kg for nine consecutive days, minimal accumulation was observed with a mean accumulation ratio of 1.37, 1.36, and 1.35, respectively. The extent of plasma exposure (AUC) was greater than dose-proportional between 0.4 and 2 mg/kg after the first dose and ninth dose.
Table 2: Mean (± standard deviation) pharmacokinetic parameters of fuzapladib sodium following nine IV doses of 0.4 mg/kg in dogs
|
C0 (μg/mL) |
3.55 ± 1.17 |
|
AUCss (hour*μg/mL) |
19.2 ± 12.7 |
|
T1/2 (hour) |
7.32 ± 4.08 |
|
Vss (L/kg) |
0.216 ± 0.070 |
|
Clss (L/h/kg) |
0.026 ± 0.009 |
C0: Back-extrapolated plasma concentration of fuzapladib sodium at time zero by a log-linear regression of first two data points following IV administration
AUCss: Area under the plasma concentration versus time curve during dosing interval at steady state
T1/2: Terminal elimination half-life
Vss: Volume of distribution at steady state
Clss: Clearance at steady state
REASONABLE EXPECTATION OF EFFECTIVENESS:
A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
PANOQUELL®-CA1 is conditionally approved pending a full demonstration of effectiveness. Additional information for Conditional Approvals can be found at www.fda.gov/animalca
The reasonable expectation of effectiveness for PANOQUELL®-CA1 for the management of clinical signs associated with acute onset of pancreatitis in dogs was based on a pilot field study.
The effectiveness of fuzapladib sodium (not commercial formulation) was demonstrated in a well-controlled pilot field study. A total of 61 client-owned dogs of various breeds between 1.8 and 15.9 years old were enrolled in the study and 36 dogs were included in the effectiveness analysis. Dogs were diagnosed with acute onset of pancreatitis based on clinical signs, clinical pathology results, and a Day 0 canine pancreatic lipase immunoreactivity (cPLI) concentration of ≥ 400 μg/L. Abdominal imaging consisting of ultrasound and/or radiographs were evaluated to exclude cases of gastrointestinal obstruction/foreign body and abdominal masses. Dogs with severe concurrent life-threatening illness other than acute pancreatitis were also excluded. Of the 25 dogs excluded from the effectiveness analysis, most of these dogs (19) began the study before the cPLI results from Day 0 were finalized and were later excluded because their cPLI results were ≤ 400 μg/L. Six dogs were excluded for other reasons.
Of the 36 dogs included in the effectiveness analysis, 17 dogs received 0.4 mg/kg fuzapladib sodium and 19 dogs received 0.1 mL/kg vehicle control (excipient lyophilized powder solubilized in 1 mL of Sterile Water for Injection, USP) IV once daily for three days. All dogs enrolled in the study received the standard of care for acute onset of pancreatitis, including fluids, nutritional support, pain medications (excluding NSAIDs), anti-emetics, and medications used to treat well-controlled pre-existing conditions. Some dogs also received parasiticides and vaccinations.
A Modified Canine Activity Index (MCAI) was used to evaluate and score the following seven clinical signs relevant in dogs with acute pancreatitis: Activity, Appetite (voluntary food intake), Vomiting, Cranial abdominal pain, Dehydration, Stool consistency, and Blood in the stool.
The primary effectiveness variable was the change in the group mean total MCAI score from Day 0 (pre-treatment) to Day 3, as assessed by the Investigator. Day 0 mean MCAI scores for the fuzapladib sodium and vehicle control groups were 8.53 and 7.68, respectively. The changes in the mean total MCAI scores from Day 0 to 3 for the fuzapladib sodium and vehicle control groups were -7.7 and -5.7, respectively. Dogs treated with fuzapladib sodium had a statistically significant reduction in MCAI scores compared to control (p = 0.0193).
TARGET ANIMAL SAFETY:
In a 9-day laboratory study, 32 healthy intact Beagle dogs (4 dogs/sex/group) aged 6 to 7 months were administered 0.4 (1X), 1.2 (3X), or 2 (5X) mg/kg PANOQUELL®-CA1 (fuzapladib sodium for injection), or saline control, by IV injection once daily for 9 days. All dogs survived to study termination. The administration of PANOQUELL®-CA1 resulted in hypertension and injection site swelling and bruising in a dose dependent manner with increased frequency in the higher dose groups.
Hypertension (systolic blood pressure values of ≥ 160 mmHg) was observed only in dogs administered PANOQUELL®-CA1 and occurred only at the end of the study. Mild thrombocytopenia of 121-169 x 103/L (reference range: 171- 361 x 103/L) was observed in two 0.4 mg/kg group dogs and one 2 mg/kg group dog on one day each. One dog in the 0.4 mg/kg group also had bruising of the injection site that coincided with the thrombocytopenia. One dog in the 2 mg/kg group had pain associated with the injection on the last day of dosing.
Focal subcutaneous hemorrhage of the injection sites was observed on gross necropsy in all groups, including control, but increased in severity in a dose dependent manner. On histopathology, observations of increased incidence and severity of dermal fibroplasia, subcutaneous inflammation, and subcutaneous hemorrhage of the injection sites were found only in dogs administered PANOQUELL®-CA1.
How Supplied
PANOQUELL®-CA1 consists of two separate vials. One vial contains 14 mg of fuzapladib sodium, 52.5 mg of D-mannitol, and 21 mg of tromethamine as sterile lyophilized powder. The second vial of 3.9 mL sterile diluent (bacteriostatic water for injection), containing 1.8% w/v benzyl alcohol, is for reconstituting the sterile lyophilized powder prior to use. No other diluent should be used.
STORAGE, HANDLING, AND DISPOSAL:
Store unopened vials at room temperature, 59° to 77°F (15° to 25°C). Store the reconstituted product at refrigerated conditions, 36° to 46°F (2° to 8°C). Use within 28 days of first puncture.
MANUFACTURED FOR:
Ishihara Sangyo Kaisha, Ltd., Osaka, Japan
DISTRIBUTED BY:
Ceva Animal Health, LLC, Lenexa, KS 66215
PANOQUELL® is a registered trademark of Ishihara Sangyo Kaisha Ltd.
PC5426A
ISK/PAN/P1/1
Revision date: 11/22
G44310B 08-BA1-v1
CPN: 1328141.0
8735 ROSEHILL ROAD, STE. 300, LENEXA, KS, 66215
| Toll-Free: | 1-800-999-0297 | |
| Toll-Free Fax: | 877-777-5138 | |
| Website: | www.ceva.us |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
