Palladia
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
10 mg, 15 mg, 50 mg
(toceranib phosphate) Tablets
Antineoplastic
For oral use in dogs only
Palladia Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
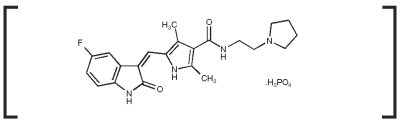
PALLADIA, a multi-kinase inhibitor targeting several receptor tyrosine kinases (RTK), is the phosphate salt of toceranib. The empirical formula is C22H25FN4O2H3O4P and the molecular weight is 494.46. The chemical name is (Z) - 5 - [(5 - Fluoro - 2 - oxo - 1,2 - dihydro - 3H - indol - 3 - ylidene)methyl] - 2,4 - dimethyl - N - (2 - pyrrolidin - 1 - ylethyl) - 1H - pyrrole - 3 - carboxamide phosphate. Toceranib phosphate is a small molecule with an indolinone chemical structure.
The chemical structure of toceranib phosphate is
Palladia Indications
PALLADIA tablets are indicated for the treatment of Patnaik grade II or III, recurrent, cutaneous mast cell tumors with or without regional lymph node involvement in dogs.
Dosage and Administration
Always provide Client Information Sheet with prescription. Administer an initial dosage of 3.25 mg/kg (1.48 mg/lb) body weight, orally every other day (see Table 1). Dose reductions of 0.5 mg/kg (to a minimum dose of 2.2 mg/kg (1.0 mg/lb) every other day) and dose interruptions (cessation of PALLADIA for up to two weeks) may be utilized, if needed, to manage adverse reactions (see Table 2 as well as Warnings and Precautions). Adjust dose based on approximately weekly veterinary assessments for the first 6 weeks and approximately every 6 weeks, thereafter. PALLADIA may be administered with or without food. Do not split tablets.Table 1. 3.25 mg/kg Dose Chart
|
Dog Body Weight |
|
Number of Tablets |
|||
|
Pounds |
Kilograms |
Dose |
10 mg |
15 mg |
50 mg |
|
11.0 - 11.8 |
5.0 - 5.3 |
15 mg |
|
1 |
|
|
11.9 - 15.2 |
5.4 - 6.9 |
20 mg |
2 |
|
|
|
15.3 - 18.5 |
7.0 - 8.4 |
25 mg |
1 |
1 |
|
|
18.6 - 22.0 |
8.5 - 10.0 |
30 mg |
|
2 |
|
|
22.1 - 25.4 |
10.1 - 11.5 |
35 mg |
2 |
1 |
|
|
25.5 - 28.7 |
11.6 - 13.0 |
40 mg |
1 |
2 |
|
|
28.8 - 32.2 |
13.1 - 14.6 |
45 mg |
|
3 |
|
|
32.3 - 35.5 |
14.7 - 16.1 |
50 mg |
|
|
1 |
|
35.6 - 38.8 |
16.2 - 17.6 |
55 mg |
1 |
3 |
|
|
38.9 - 42.3 |
17.7 - 19.2 |
60 mg |
1 |
|
1 |
|
42.4 - 45.6 |
19.3 - 20.7 |
65 mg |
|
1 |
1 |
|
45.7 - 50.7 |
20.8 - 23.0 |
70 mg |
2 |
|
1 |
|
50.8 - 59.3 |
23.1 - 26.9 |
80 mg |
|
2 |
1 |
|
59.4 - 65.9 |
27.0 - 29.9 |
95 mg |
|
3 |
1 |
|
66.0 - 71.2 |
30.0 - 32.3 |
100 mg |
|
|
2 |
|
71.3 - 76.3 |
32.4 - 34.6 |
110 mg |
1 |
|
2 |
|
76.4 - 79.6 |
34.7 - 36.1 |
115 mg |
|
1 |
2 |
|
79.7 - 84.7 |
36.2 - 38.4 |
120 mg |
2 |
|
2 |
|
84.8 - 94.8 |
38.5 - 43.0 |
130 mg |
|
2 |
2 |
|
94.9 - 105.0 |
43.1 - 47.6 |
150 mg |
|
|
3 |
|
105.1 - 110.0 |
47.7 - 49.9 |
160 mg |
1 |
|
3 |
|
110.1 - 113.5 |
50.0 - 51.5 |
165 mg |
|
1 |
3 |
|
113.6 - 118.6 |
51.6 - 53.8 |
170 mg |
2 |
|
3 |
|
118.7 - 128.8 |
53.9 - 58.4 |
180 mg |
|
2 |
3 |
|
128.9 - 138.9 |
58.5 - 63.0 |
200 mg |
|
|
4 |
|
139.0 - 144.0 |
63.1 - 65.3 |
210 mg |
1 |
|
4 |
|
144.1 - 157.6 |
65.4 - 71.5 |
215 mg |
|
1 |
4 |
|
157.7 - 173.1 |
71.6 - 78.5 |
250 mg |
|
|
5 |
|
173.2 - 177.9 |
78.6 - 80.7 |
260 mg |
1 |
|
5 |
|
178.0 - 191.6 |
80.8 - 86.9 |
265 mg |
|
1 |
5 |
|
191.7 - 220.5 |
87.0 - 100.0 |
300 mg |
|
|
6 |
Table 2: Dose Modification Based on Toxicity Observed
|
Toxicity |
Dose Adjustment |
|
Neutropenia |
|
|
>1000/µL |
Maintain dose level |
|
≤1000/µL or neutropenic fever or infection |
Stop drug until >1000/µL and clinical signs normal; then decrease dose by 0.5 mg/kg |
|
Renal Toxicities (Creatinine) |
|
|
<2.0 mg/dL |
Maintain dose level |
|
≥2.0 mg/dL |
Stop drug until <2.0 mg/dL then decrease dose by 0.5 mg/kg |
|
Albumin |
|
|
<1.5 g/dL |
Stop drug until >2.5 g/dL then decrease dose by 0.5 mg/kg |
|
Hematocrit |
|
|
<26% |
Stop drug until >30% then decrease dose by 0.5 mg/kg |
|
Diarrhea |
|
|
<4 watery stools/day for less than 2 days |
Maintain dose level and institute supportive care |
|
≥4 watery stools/day or ≥ 2 days |
Stop drug until formed stools and institute supportive care. When dosing is resumed, decrease dose by 0.5 mg/kg |
|
GI Bleeding |
|
|
Fresh blood in stool or black tarry stool for > 2 days or frank hemorrhage or blood clots in stool. |
Stop drug and institute supportive care until resolution of all clinical signs of blood in stool, then decrease dose by 0.5 mg/kg. |
Contraindications
Do not use in dogs used for breeding, or for pregnant or lactating bitches (see Clinical Pharmacology).
Warnings
PALLADIA may cause vascular dysfunction which can lead to edema and thromboembolism, including pulmonary thromboembolism. Discontinue drug until clinical signs and clinical pathology have normalized. To assure vasculature homeostasis, wait at least 3 days after stopping drug before performing surgery (see Adverse Reactions).
Serious and sometimes fatal gastrointestinal complications including gastrointestinal perforation have occurred rarely in dogs treated with PALLADIA (see Adverse Reactions). If gastrointestinal ulceration is suspected, stop drug administration and treat appropriately.
Human Warnings:
NOT FOR USE IN HUMANS. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Children should not come in contact with PALLADIA. Keep children away from feces, urine, or vomit of treated dogs. To avoid exposure to drug, wash hands with soap and water after administering PALLADIA and wear protective gloves to prevent direct contact with feces, urine, vomit, and broken or moistened PALLADIA tablets. Place all waste materials in a plastic bag and seal before general disposal. If eyes are accidentally exposed to the drug, rinse eyes with water immediately. In case of accidental ingestion by a person, seek medical advice immediately, show the package insert or label to the physician. Gastrointestinal discomfort such as vomiting or diarrhea may occur if this drug is accidentally ingested.
Pregnant women, women who may become pregnant, or nursing mothers should pay special attention to these handling precautions. (See handling instructions above.) PALLADIA, like other drugs in its class, prevents the formation of new blood vessels in tumors. In a similar manner, PALLADIA may affect blood vessel formation in the developing fetus and may harm an unborn baby (cause birth defects). For pregnant women, accidental ingestion of PALLADIA may have adverse effects on pregnancy.
Precautions
Temporarily discontinue the use of PALLADIA if anemia, azotemia, hypoalbuminemia, and hyperphosphatemia occur simultaneously. Resume treatment at a dose reduction of 0.5 mg/kg after 1 to 2 weeks when values have improved and albumin is >2.5 g/dL. Temporary treatment interruptions may be needed if any one of these occurs alone: hematocrit <26%, creatinine ≥2.0 mg/dL or albumin <1.5 g/dL. Then resume treatment at a dose reduction of 0.5 mg/kg once the hematocrit is >30%, the creatinine is <2.0 mg/dL, and the albumin is >2.5 g/dL.
Temporarily discontinue the use of PALLADIA if neutrophil count is ≤1000/µL. Resume treatment after 1 to 2 weeks at a dose reduction of 0.5 mg/kg, when neutrophil count has returned to >1000/µL. Further dose reductions may be needed if severe neutropenia reoccurs.
The presence of systemic mast cell tumor prior to treatment may predispose a dog to clinically significant mast cell degranulation with possible severe systemic adverse reactions when treated with PALLADIA. Attempts should be made to rule out systemic mastocytosis prior to initiation of treatment with PALLADIA.
PALLADIA has been associated with severe diarrhea or GI bleeding that requires prompt treatment. Dose interruptions and dose reductions may be needed depending upon the severity of clinical signs. (See Table 2 in Dosage and Administration.)
Use non-steroidal anti-inflammatory drugs with caution in conjunction with PALLADIA due to an increased risk of gastrointestinal ulceration or perforation.
PALLADIA is metabolized in the liver. Co-administration of PALLADIA with strong inhibitors of the CYP3A4 family may increase PALLADIA concentrations. The effect of concomitant medications that may inhibit the metabolism of PALLADIA has not been evaluated. Drug compatibility should be monitored in patients requiring concomitant medications.
The safe use of PALLADIA has not been evaluated in dogs less than 24 months of age or weighing less than 5 kg.
Adverse Reactions
A US clinical field study comprised of a 6-week masked phase, followed by an open-label phase, evaluated the safety and effectiveness of PALLADIA in 151 client-owned dogs that had Patnaik grade II or III, recurrent, cutaneous mast cell tumors with or without regional lymph node involvement. The most common adverse reactions reported during the masked phase are summarized in Table 3; those reported during the entire study (masked phase combined with the open-label phase) are summarized in Table 4.
Table 3. Summary of the most common adverse reactions during the masked phasea
|
|
Placebo (n = 64) |
PALLADIA (n = 87) |
||
|
Adverse Reaction |
Any Gradeb |
Grade 3 or 4b |
Any Gradeb |
Grade 3 or 4b |
|
Diarrhea |
26.6% |
3.1% |
46.0% |
6.9% |
|
Anorexia |
31.3% |
6.3% |
39.1% |
6.9% |
|
Lethargy |
29.7% |
3.1% |
35.6% |
4.6% |
|
Vomiting |
32.8% |
6.3% |
32.2% |
9.2% |
|
Lameness |
9.4% |
0.0% |
17.2% |
0.0% |
|
Weight loss |
3.1% |
0.0% |
14.9% |
1.1% |
|
Blood in stool/GI bleed/hemorrhagic diarrhea |
3.1% |
0.0% |
12.6% |
2.3% |
|
Musculoskeletal disorder |
6.3% |
0.0% |
11.5% |
1.1% |
|
Dehydration |
4.7% |
0.0% |
9.2% |
2.3% |
|
Dermatitis |
9.4% |
1.6% |
9.2% |
0.0% |
|
Pruritus |
4.7% |
0.0% |
9.2% |
0.0% |
|
Tachypnea |
4.7% |
0.0% |
8.0% |
1.1% |
|
Localized pain |
4.7% |
0.0% |
8.0% |
0.0% |
|
Nausea |
3.1% |
0.0% |
8.0% |
1.1% |
|
General pain |
4.7% |
1.6% |
6.9% |
0.0% |
|
Polydipsia |
7.8% |
0.0% |
6.9% |
0.0% |
|
Pyrexia |
3.1% |
0.0% |
5.7% |
2.3% |
|
Flatulence |
3.1% |
0.0% |
5.7% |
0.0% |
|
Pigmentation disorder |
1.6% |
0.0% |
5.7% |
0.0% |
|
Laboratory Abnormality |
Any Gradec |
Grade 3 or 4c |
Any Gradec |
Grade 3 or 4c |
|
Neutropenia |
6.3% |
0.0% |
46.0% |
0.0% |
|
Thrombocytopenia |
20.3% |
0.0% |
24.1% |
0.0% |
|
Increased alanine aminotransferase |
21.9% |
4.7% |
24.1% |
1.1% |
|
Hypoalbuminemia |
7.8% |
0.0% |
12.6% |
0.0% |
|
Decreased hematocrit |
7.8% |
0.0% |
5.7% |
3.4% |
|
Hyperbilirubinemia |
1.6% |
1.6% |
5.7% |
0.0% |
|
Increased creatinine |
4.7% |
0.0% |
5.7% |
0.0% |
|
Urinary tract infection |
1.6% |
0.0% |
5.7% |
0.0% |
a The mean time on study during the masked phase was 37.0 days for PALLADIA-treated dogs (median, 42.0 days) and 27.6 days for placebo-treated dogs (median, 21.0 days); no adjustments were made in the statistical comparisons for this disparity.
b Investigators assigned severity grade of 1, 2, 3 or 4 (1 - least severe; 4 - most severe).
c Grading of laboratory abnormalities was based on the National Cancer Institute’s Common Toxicity Criteria guideline adapted for canines (1 - least severe; 4 - most severe).
Table 4. Summary of the most common adverse reactions during the study (masked phase combined with the open-label phase)a
|
|
PALLADIA (n = 145) a |
|
|
Adverse Reactions |
Any Gradeb |
Grade 3 or 4b |
|
Diarrhea |
58.6% |
8.3% |
|
Anorexia |
49.7% |
8.3% |
|
Vomiting |
47.6% |
9.7% |
|
Lethargy |
39.3% |
4.1% |
|
Lameness |
22.8% |
0.0% |
|
Weight loss |
21.4% |
2.8% |
|
Blood in stool/GI bleed/hemorrhagic diarrhea |
18.6% |
2.8% |
|
Dehydration |
15.2% |
2.1% |
|
Pruritus |
12.4% |
0.0% |
|
Pigmentation disorder |
11.7% |
0.0% |
|
Dermatitis |
11.0% |
0.0% |
|
Musculoskeletal disorder |
11.0% |
0.0% |
|
General pain |
8.3% |
0.0% |
|
Otitis externa |
8.3% |
0.0% |
|
Tachypnea |
8.3% |
0.0% |
|
Nausea |
7.6% |
1.4% |
|
Polydipsia |
7.6% |
0.0% |
|
Pyrexia |
6.9% |
2.8% |
|
Arthritis |
6.2% |
0.0% |
|
Localized edema |
6.2% |
0.0% |
|
Bacterial skin infection |
5.5% |
0.0% |
|
Conjunctivitis |
5.5% |
0.0% |
|
Laboratory Abnormality |
Any Gradec |
Grade 3 or 4c |
|
Neutropenia |
44.8% |
1.4% |
|
Hypoalbuminemia |
28.3% |
1.4% |
|
Thrombocytopenia |
28.3% |
2.1% |
|
Increased alanine aminotransferase |
27.6% |
4.1% |
|
Decreased hematocrit |
11.0% |
2.8% |
|
Increased creatinine |
13.8% |
1.4% |
|
Hyperbilirubinemia |
6.9% |
0.0% |
|
Urinary tract infection |
7.6% |
0.0% |
a The duration of treatment with PALLADIA ranged from 2 to 812 days (mean, 144 days; median, 68 days). All dogs received at least 1 dose of PALLADIA.
b Investigators assigned severity grade of 1, 2, 3 or 4 (1 - least severe; 4 - most severe).
c Grading of laboratory abnormalities was based on the National Cancer Institute’s Common Toxicity Criteria guideline adapted for canines (1 - least severe; 4 - most severe).
Other adverse events were reported but occurred in < 5% of dogs. Any individual dog may have had multiple adverse events.
There were 5 deaths during this study that were possibly drug related. Pathology findings generally revealed evidence of vascular dysfunction including pulmonary thromboembolism (post-operative); multi-organ failure associated with vasculitis and thrombosis; vascular thrombosis with disseminated intravascular coagulopathy (DIC) and pancreatitis; and vasculitis with DIC. One dog died secondary to gastric perforation; the duration of treatment with PALLADIA was 221 days and there was no evidence of mast cell tumor at necropsy. These deaths occurred in the presence or absence of gross-disease; treatment durations ranged from 18 to 221 days.
The relationship of the following deaths to drug are unknown. One dog, first treated for 3 weeks with a placebo, died of unknown cause 7 days after initiation of PALLADIA therapy. Another dog died of unknown cause 92 days after initiation of PALLADIA therapy. No necropsy was conducted in either dog.
Twenty seven dogs developed some form of gastrointestinal bleeding with 2.8% of dogs having severe bleeding. One dog developed gastric ulceration which was possibly drug related. Three dogs died from gastric (1 dog) or duodenal (2 dogs) perforations during the study. One dog with a duodenal perforation received only 1 dose of study drug and, therefore, was not considered drug related.
Seven dogs developed nasal depigmentation within the first few weeks of treatment. Eleven dogs developed coat color or skin changes during the study. Two of these dogs had complete coat color changes from fawn to white and from deep red to blonde. Seven dogs experienced alopecia.
There is a drug related effect on body weight: 20.0% of dogs had >13% weight loss in the masked plus open-label phase attributable to drug. Of these, 5 dogs had >25% weight loss.
Three dogs had seizure-like activity while on study drug. It can not be determined if these were drug related.
Two dogs developed epistaxis that was not associated with thrombocytopenia. Another dog developed epistaxis with concurrent disseminated intravascular coagulopathy.
For a copy of the Safety Data Sheet (SDS) or to report adverse events call Zoetis at 1-888-963-8471.
Information for Dog Owners:
Always provide Client Information Sheet with prescription and review with owners. Owners should be advised on possible adverse reactions and when to stop drug and call the veterinarian. Owners should be advised of the handling instructions.
Clinical Pharmacology
Mechanism of Action: Toceranib phosphate is a small molecule that has both direct antitumor and antiangiogenic activity. In non-clinical pharmacology studies, toceranib selectively inhibited the tyrosine kinase activity of several members of the split kinase receptor tyrosine kinase (RTK) family, some of which are implicated in tumor growth, pathologic angiogenesis, and metastatic progression of cancer. Toceranib inhibited the activity of Flk-1/KDR tyrosine kinase (vascular endothelial growth factor receptor, VEGFR2), platelet-derived growth factor receptor (PDGFR), and stem cell factor receptor (Kit) in both biochemical and cellular assays. Toceranib has been shown to exert an antiproliferative effect on endothelial cells in vitro. Toceranib treatment can induce cell cycle arrest and subsequent apoptosis in tumor cell lines expressing activating mutations in the split kinase RTK, ckit. Canine mast cell tumor growth is frequently driven by activating mutations in c-kit.1,2
Other compounds in the antiangiogenesis class of antineoplastic agents are known to increase embryolethality and fetal abnormalities. As angiogenesis is a critical component of embryonic and fetal development, inhibition of angiogenesis following administration of PALLADIA should be expected to result in adverse effects on the pregnancy in the bitch.
Pharmacokinetics
Following intravenous administration, the pharmacokinetics of toceranib is characterized by a very large volume of distribution (>20 L/kg, indicating partitioning into tissues), a terminal elimination half-life of about 16 hrs, and a clearance of >1 L/hr/kg. With a regimen of 3.25 mg free base equivalent (fbe)/kg doses of toceranib administered by tablet orally every other day for 2 weeks (7 doses), the pharmacokinetic parameters of toceranib in plasma in healthy Beagle dogs (between 7.2 - 12.5 kg) are shown in the table below.
Table 5: Pharmacokinetic Parameters
|
Pharmacokinetic Parameters (Mean + 1SD) |
Total (n=11;6M, 5F) |
Total (n=10; 5M, 5F) |
|
Elimination half-life, t1/2 (h) |
16.4 ± 3.6 |
17.2 ± 3.9 |
|
Time to maximum plasma concentration, Tmax (h) |
5.3 ± 1.6 |
6.2 ± 2.6 |
|
Maximum plasma concentration, Cmax (ng/mL) |
86 ± 22 |
109 ± 41 |
|
Cmin (ng/mL) a, b |
12.7 ± 6.0 |
18.7 ± 8.3 |
|
Area under the plasma concentration time-curve, AUC0-48 (ng•h/mL) a |
1833 ± 508 |
2635 ± 939 |
a Dose-normalized value (adjusted to 3.25 mg/kg dose)
b Cmin is the concentration at 48 h post-dose, which corresponds to the dose interval.
Oral bioavailability of toceranib is 77%. PALLADIA is highly protein bound at 91% to 93%.
It should be noted that despite the homogeneity of subjects included in this study, large between-subject variability was observed. Regardless of the route of administration, linear pharmacokinetics has been observed at doses up to 5 mg/kg twice daily. Using an in vitro hepatocyte and liver microsome test system, the Z isomer was found to be metabolized to the N-oxide derivative of toceranib in dogs, humans, cats, and rats. Although a small gender difference was observed in the in vitro study (81% conversion in male dogs, 56% conversion in female dogs) no differences in toceranib pharmacokinetics was observed in vivo. The effects of renal impairment, hepatic impairment or breed on the pharmacokinetics of toceranib have not been investigated.
Effectiveness
The effectiveness and safety of PALLADIA oral tablets for the treatment of mast cell tumors was evaluated in a randomized, placebo-controlled, double-masked, multicenter clinical field study. The purpose of this study was to evaluate the effectiveness and safety of PALLADIA in the treatment of mast cell tumors in dogs that had recurrent measurable disease after surgery and to evaluate objective response (complete or partial response). PALLADIA treatment was compared to placebo treatment using response rates at the end of the 6-week masked phase. Response rates were determined using the National Cancer Institute’s Response Evaluation Criteria in Solid Tumors Guideline3 which was modified specifically for the evaluation of canine mast cell tumors.
One-hundred-fifty-three dogs were randomly assigned to treatment with either 3.25 mg/kg PALLADIA (n = 88) or placebo (n = 65) orally, every other day for 6 weeks, or until disease progression or withdrawal from the study for another cause. Treatment was unmasked at the time of disease progression: dogs receiving placebo were then offered crossover to open-label PALLADIA; dogs receiving PALLADIA were discontinued from the study. Dogs were required to have Patnaik grade II or III, recurrent, cutaneous mast cell tumors with or without regional lymph node involvement. At least 1 tumor had to be at least 20 mm in diameter. Dogs had a limit of 1 completed radiation protocol and a limit of 1 prior systemic chemotherapy regimen. Dogs with evidence of systemic mast cell tumor were excluded. Treatment with systemic corticosteroids during the study or within 14 days prior to study initiation was not permitted. If needed to manage adverse reactions, dose interruptions (cessation of PALLADIA for up to 2 weeks) were prescribed and/or dosage was reduced to as low as 2.2 mg/kg.
The effectiveness analysis showed a statistically significant advantage for PALLADIA over placebo in the primary effectiveness endpoint of objective response at the end of the six week masked phase. Objective response is complete response + partial response. Partial response is ≥ 30% decrease in the sum of the longest diameter of target lesions, taking as reference the baseline sum, non-progression of non-target lesions and appearance of no new lesions.
Mast Cell Tumor - Primary Effectiveness Endpoint Results
|
Effectiveness Parameter |
Placebo (n = 63) |
PALLADIA (n = 86) |
P-value |
|
Objective Response Rate * |
7.9% |
37.2% |
< 0.001 |
* The difference in objective response rate between groups was not significantly associated with tumor burden (presence vs. absence of regional lymph node involvement) or tumor grade (P > 0.05).
During the study, PALLADIA was administered concomitantly with other medications such as antimicrobials, H-2 receptor blockers, antihistamines, anti-emetics, non-steroidal anti-inflammatory drugs, locally-acting anti-ulcer medications, opiate gastrointestinal motility modifiers, opioids, vaccines, anthelmintics, antiparasitics, and topical/ophthalmic/otic corticosteroid preparations. During the open-label phase only, 5 dogs received a brief course of short-acting corticosteroids.
Animal Safety:
In the target animal safety study presented below, PALLADIA was demonstrated to have a narrow margin of safety; dogs being treated with PALLADIA should be monitored for adverse reactions which may indicate a dose adjustment is required. Two dogs in the 6 mg/kg group were euthanized for clinical toxicities on Days 23 and 27 of the study, respectively.
Toceranib was administered orally to 20 male and 20 female adult Beagle dogs (approximately 2 years of age) at doses of 0 mg/kg (placebo, 12 dogs), 2 mg/kg (0.5X, 8 dogs), 4 mg/kg (1X, 12 dogs), or 6 mg/kg (1.5X, 8 dogs) once every other day for 13 consecutive weeks without dose interruption. Toceranib caused weight loss, decreased feed consumption, pancreatic, gonadal, adrenal, muscle, and hematopoietic changes.
Feed consumption was decreased in the 6 mg/kg group compared to placebo, with the largest difference in means occurring at Day 35. Decrease in body weights in the 4 mg/kg group were seen at Day 31 and in the 6 mg/kg group at Day 15 compared with placebo and continued through the study. Dose related lameness, observed almost exclusively in the hind limbs, and limb pain was greater in all treatment groups as compared to placebo, with the 6 mg/kg group demonstrating the highest incidence. Stiffness and weakness were noted to occur almost exclusively in the 6 mg/kg group. Redness of oral mucosa was observed in all treatment groups. One dog in the 4 mg/kg group had oral ulcerations and one dog in the 6 mg/kg group had skin ulcerations, both with bacterial infections present. Diarrhea or soft stool were seen in all four groups.
Hematology analyses showed decreases in hematocrit, hemoglobin, and erythrocyte count and a decrease in reticulocyte count in the 4 and 6 mg/kg groups that tended to recover sufficiently to limit further erythrocyte count decreases. White blood cell counts were significantly lower across the study in all treated groups compared to placebo, primarily due to a decrease in neutrophils. Lymphocytes decreased to a lesser degree, especially at the low dose. Eosinophils and basophils showed marked, persistent decreases. Monocytes were not affected.
Platelet counts increased slightly in 4 and 6 mg/kg groups. Increases were observed in fibrinogen in the 4 and 6 mg/kg group.
Increases were observed in aspartate aminotransferase, creatine kinase, and serum phosphorus concentrations in the 4 and 6 mg/kg groups. Increases in alkaline phosphatase were seen in the 6 mg/kg group. An increase in amylase was seen in one dog in each of the treatment groups. An increase in serum potassium was seen in one dog in the 6 mg/kg group. Increases in lactate dehydrogenase and globulins were observed in the 6 mg/kg group.
Treatment-related microscopic changes included slight to marked reduction in cellularity of sternal and femoral bone marrow. There was a corresponding mild extramedullary hematopoiesis, mainly erythropoiesis, in the spleen. In the pancreas, dose-related slight to moderate acinar degranulation, characterized by diffuse loss of zymogen granules, occurred. In the adrenal glands, minimal cortical congestion/hemorrhage occurred at all doses, with suggestive dose-relationship. Adrenal cortical vacuolation was noted with low frequency in all groups. Dose related changes were noted in reproductive organs of both sexes. Males showed a dose-related germ cell depletion, tubular vacuolation, and reductions in numbers of mature spermatozoa. In females, ovaries showed a reduced incidence of mature/regressing corpora lutea and an increased incidence of small follicles.
Two dogs (one male, one female) in the 6 mg/kg group were euthanized for treatment-related clinical toxicities on Days 23 and 27 of the study, respectively. Onset of the terminal syndrome was seen as markedly reduced feed intake and melena. Over the following 9 days, the decreased feed intake progressed to near-complete anorexia and hematochezia appeared. Weight loss, lethargy, hindlimb lameness and weakness were observed. The following clinical pathology results are consistent with changes seen in the other dogs in the 6 mg/kg group as well as changes due to the dogs’ debilitated conditions just prior to euthanasia. Both dogs had increases in total protein, globulins, phosphorus, cholesterol, triglycerides and fibrinogen. One dog had pancytopenia, decreased hematocrit, hemoglobin, reticulocytes, albumin, and PT and increased bands. Hematuria was also present. The other dog also had decreased lymphocytes, eosinophils, chloride, and sodium and increases in RBC, hematocrit, hemoglobin, platelets, ALP, amylase, creatinine, BUN, magnesium, potassium, and total bilirubin. Clotting profile showed a decreased PT and increased in PTT in both dogs. These dogs showed lymphoid depletion in lymph nodes, thymus, and gut-associated lymphatic tissues and mild to marked gastrointestinal lesions in addition to the microscopic findings described in animals surviving to the end of the study. These two dogs also had lesions in the gastrointestinal tract, kidneys, pancreas, pituitary gland and adrenal glands.
Storage Conditions: Store at controlled room temperature 20° to 25° C (68° to 77° F).
How Supplied
PALLADIA tablets contain 10 mg, 15 mg, or 50 mg of toceranib as toceranib phosphate per tablet. The tablets are packaged in 30 count bottles.References
1 London CA, Hannah AL, Zadovoskaya R, et al. Phase I Dose-Escalating Study of SU11654, a Small Molecule Receptor Tyrosine Kinase Inhibitor, in Dogs with Spontaneous Malignancies. Clinical Cancer Research 9(7):2755-2768; 2003
2 Pryer NK, Lee LB, Zadovoskaya R, et al. Proof of Target for SU11654: Inhibition of KIT phosphorylation in Canine Mast Cell Tumors. Clinical Cancer Research 9(15):5729-5734; 2003
3 http://ctep.cancer.gov/protcolDevelopment/
Approved by FDA under NADA # 141-295
Manufactured by: Pfizer Inc, Ascoli, Italy
Product of the United Kingdom
Distributed by:
Zoetis Inc., Kalamazoo, MI 49007
Revised: June 2023
PAA210853
CPN: 3690372.3
333 PORTAGE STREET, KALAMAZOO, MI, 49007
| Telephone: | 269-359-4414 | |
| Customer Service: | 888-963-8471 | |
| Website: | www.zoetis.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
