Lincomycin-Spectinomycin Water Soluble Powder
This treatment applies to the following species: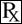 Company: Huvepharma
Company: Huvepharma
ANTIBACTERIAL AND ANTIMYCOPLASMAL
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
FOR USE IN CHICKENS ONLY
This packet contains:
|
Lincomycin hydrochloride, equivalent to lincomycin |
16.7 grams |
|
Spectinomycin dihydrochloride pentahydrate, equivalent to spectinomycin |
33.3 grams |
|
Total antibiotic activity |
50.0 grams |
Approved by FDA under ANADA # 200-407
Directions For Use
Lincomycin-Spectinomycin Water Soluble Powder Indications and Usage
For use in chickens up to 7 days of age as an aid in the control of: Airsacculitis caused by either Mycoplasma synoviae or Mycoplasma gallisepticum susceptible to lincomycin-spectinomycin.Complicated Chronic Respiratory Disease (Air Sac Infection) caused by Escherichia coli and M. gallisepticum susceptible to lincomycin-spectinomycin.
Lincomycin-Spectinomycin Water Soluble Powder Dosage And Administration
Dosage: Provide 2 grams (g) antibiotic activity per gallon of drinking water. Administer as the sole source of water for the first 5 to 7 days of life.
Administration:
|
Amount of Lincomycin-Spectinomycin |
Amount of Drinking Water |
|
1 packet |
25 gallons |
|
For proportioners delivering 1 ounce of solution per gallon of drinking water, dissolve contents of 5 packets in each gallon of proportioner solution. |
|
IMPORTANT: Chickens should consume water at the following approximate rate to insure intake of the required dose of lincomycin-spectinomycin indicated:
|
Broilers and Layer Replacements (Light and Heavy) |
||
|
Age |
Daily Water Intake |
Dosage |
|
1 |
5 |
50-65 |
PRECAUTION: Discard medicated drinking water daily and replace with fresh medicated drinking water.
Warning
Not For Human UseKeep Out of Reach of Children
Restricted Drug (California) - Use Only as Directed
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at 1-877-994-4883 or www.huvepharma.us. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Restricted Drug (California) - Use Only as Directed
Store at Controlled Room Temperature, 20° to 25°C (68° to 77°F)
Manufactured For:
Huvepharma, Inc. Peachtree City, GA 30269
|
NET WEIGHT: 2.65 oz. (75 grams) |
Rev. 04-2021 L-4248-01 |
|
Net Contents: 12 x 2.65 oz. (75 g) Packets |
MF# L-4248-01C Rev. 04-2021 |
CPN: 1309073.0
525 WESTPARK DR., SUITE 230, PEACHTREE CITY, GA, 30269
| Telephone: | 770-486-7212 | |
| Toll-Free: | 877-994-4883 | |
| Fax: | 770-486-7217 | |
| Website: | www.huvepharma.us |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
