Ingelvac 3FLEX (Canada)
This treatment applies to the following species:Porcine Reproductive & Respiratory Syndrome-Circovirus Vaccine
Reproductive & Respiratory Form, Type 2, Modified Live Virus, Killed Baculovirus Vector
Mycoplasma Hyopneumoniae Bacterin
For animal use only.
Ingelvac 3FLEX Indications
This product has been shown to be effective for the vaccination of healthy swine 3 weeks of age or older against porcine circovirus type 2 (PCV2), Mycoplasma hyopneumoniae, and respiratory form of porcine reproductive and respiratory syndrome (PPRS) virus. The duration of immunity is at least 4 months against the respiratory form of PRRS disease, at least 4 months against PCV2, and at least 26 weeks against Mycoplasma hyopneumoniae. For more information regarding efficacy and safety data go to productdata.aphis.usda.gov.
The product contains PCV2a, and has been shown to be effective against lymphoid depletion, inflammation and colonization of lymphoid tissue.
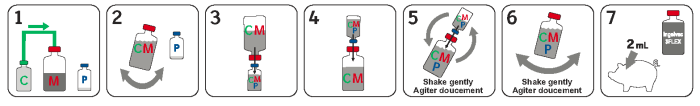
Directions and dosage: Shake each bottle of liquid product before use. Transfer entire contents of Ingelvac CircoFLEX® 1 into Ingelvac MycoFLEX® 2 headspace bottle and gently shake (Diagram 1 & 2). Next transfer a portion of the mixed vaccine from Ingelvac MycoFLEX® 2 bottle into Ingelvac® PRRS MLV 3 bottle (Diagram 3). The amount transferred needs to be enough to rehydrate the Ingelvac® PRRS MLV 3 product. Rotate the Ingelvac® PRRS MLV 3 and Ingelvac MycoFLEX® 2 bottles while still attached (Diagram 4), and then transfer rehydrated product back into the Ingelvac MycoFLEX® 2 bottle (Diagram 5). Shake well and use immediately (Diagram 6 & 7). Rehydrate only with the accompanying vaccines, do not mix other materials.
Using aseptic technique, inject a single 2 mL dose of vaccine intramuscularly.
Precautions
Store at 2-8°C. Do not freeze. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Do not mix with other products, except as specified on the carton. In case of anaphylactoid reaction, administer epinephrine. Not for use in pregnant swine or boars. For use in PRRS positive herds only. PRRS vaccine virus may be shed and transmitted to other populations of swine in contact with vaccinated swine. The duration of potential vaccine virus transmission may vary. Use of the vaccine in herds intended to remain PRRS virus seronegative is contraindicated. Introduction of vaccinated pigs into herds intended to remain PRRS virus seronegative is contraindicated. In case of human exposure, contact a physician. Inactivate unused contents before disposal.Preservative: Neomycin
INGELVAC®, 3FLEX®, INGELVAC MYCOFLEX®, and INGELVAC CIRCOFLEX® are registered trademarks of Boehringer Ingelheim Vetmedica GmbH, used under license.
Manufactured by:
Boehringer Ingelheim Animal Health USA Inc., St. Joseph, Missouri 64506 USA
Tel.: 1-888-637-4251
VLN/PCN 124/49K9.R0
Manufactured for:
Boehringer Ingelheim Animal Health Canada Inc., Burlington ON L7L 5H4
|
50 doses/Mix to 100 mL |
This package contains one 50 dose vial of Ingelvac CircoFLEX® 1, one 50 dose vial of Ingelvac MycoFLEX® 2, and one 50 dose vial of Ingelvac® PRRS MLV 3. Ingelvac MycoFLEX® 2 is supplied in a headspace bottle (HSB) with extra capacity for the purpose of aseptic mixing. |
129907-04 |
CPN: 1182223.1
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
