GleptoForte (gleptoferron)
This treatment applies to the following species:Approved by FDA under ANADA # 200-587
200 mg/mL of iron
Solution for intramuscular injection
For the prevention and treatment of Baby Pig Anemia due to iron deficiency
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Description
GleptoForte® is a sterile aqueous colloidal solution of gleptoferron, a macromolecular complex of ferric hydroxide and dextran glucoheptonic acid. Each milliliter contains 200 mg of elemental iron. The solution contains 0.5% phenol as preservative.
ACTIONS: GleptoForte® contains gleptoferron which provides a rapidly absorbed and readily utilizable form of iron for the prevention and treatment of baby pig anemia.
GleptoForte (gleptoferron) Indications
GleptoForte® is recommended for both the routine prevention and treatment of baby pig anemia due to iron deficiency.
NOTICE: Organic iron preparations injected intramuscularly into pigs beyond 4 weeks of age may cause staining of the muscle tissue.
Dosage and Administration
GleptoForte® is for intramuscular use only.Prevention: 1 mL (200 mg iron) per pig on or before 3 days of age.
Treatment: 1 mL (200 mg iron) per pig as soon as signs of deficiency appear.
 |
WITHDRAWAL PERIODS: No withdrawal period is required when used according to labeling. |
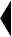 |
ANIMAL SAFETY WARNINGS AND PRECAUTIONS: Occasionally pigs may show a reaction to injectable iron, clinically characterized by prostration with muscular weakness. In extreme cases, death may occur.
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact CEVA at 1-800-999-0297.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/AnimaIVeterinary/SafetyHealth.
HOW TO INJECT:
What to use
1. GleptoForte® (gleptoferron injection)
2. Ordinary 10 mL syringe with 18-gauge needle or smaller, or dosage delivery device with 4-gauge draw-off spike or smaller
3. Disinfectant (rubbing alcohol or tincture of iodine)
Where to use
GleptoForte® is for intramuscular use. The preferred site of injection is the ham muscle. Push the needle directly into the ham to approximately a one-half (1/2) inch depth. Avoid injection into a blood vessel. This is best avoided by pulling back slightly on the plunger after the needle is seated in the ham. If properly located, resistance will be offered to this effort. If the plunger is pulled back easily, remove and relocate the needle.
Step 1
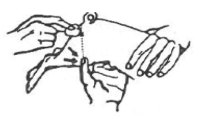
The pig is held under-arm, between knees, or by an assistant. Disinfect the injection site. Pull down skin and fat with thumb as shown. Approximately halfway along a line between the anus and the thumb is the best injection site.
Step 2
Before injecting GleptoForte®, point the needle upward and gently press the plunger to expel all air from syringe. The needle is inserted at right-angle to the skin into the ham approximately a one-half (1/2) inch depth and the injection made.
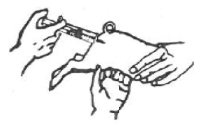
Step 3
Use a new needle between each stopper puncture. Protect the needle from contamination and disinfect between animal injections.
How Supplied
GleptoForte® (gleptoferron) is available in 100 mL and 250 mL multi-dose vials.
Storage
Store at 20°C - 25°C (68°F - 77°F). Do not freeze. Use within 28 days of first puncture and puncture a maximum of seventy-five (75) times with an 18-gauge needle or five (5) times with a dosage delivery device. If using a needle larger than 18-gauge or a draw-off spike larger than
4-gauge, discard any remaining product immediately after use.
GleptoForte® is a registered trademark of Ceva Santé Animale S.A.
Manufactured in Brazil
MANUFACTURED FOR:
Ceva Animal Health, LLC, Lenexa, KS 66215
1-800-999-0297
|
Net Contents: |
|
|
100mL Multi-dose, sterile vial. |
E59410B-US 20-K1-v1 |
|
250mL Multi-dose, sterile vial. |
E59430B 20-T1-v1 |
CPN: 1328182.0
8735 ROSEHILL ROAD, STE. 300, LENEXA, KS, 66215
| Toll-Free: | 1-800-999-0297 | |
| Toll-Free Fax: | 877-777-5138 | |
| Website: | www.ceva.us |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
