The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
GiardiaVax
This page contains information on GiardiaVax for veterinary use.The information provided typically includes the following:
- GiardiaVax Indications
- Warnings and cautions for GiardiaVax
- Direction and dosage information for GiardiaVax
Giardiavax
This treatment applies to the following species:Giardia Lamblia Vaccine
Killed Protozoa
For Use In Dogs Only
For subcutaneous vaccination of healthy dogs, 8 weeks of age or older, as an aid in the prevention of disease and shedding caused by Giardia lamblia infections.
Giardiasis is a health concern for people and dogs. While Giardia infection is a recognized zoonotic disease, the role that the dog assumes in human disease is not well established. GiardiaVax® has been proven to prevent clinical disease caused by Giardia lamblia infection in dogs and to significantly reduce the incidence, severity and duration of cyst shedding. Subsequent to Giardia lamblia exposure, some vaccinates may shed, therefore, proper hygiene and sanitation practices should be implemented.
Dose
Dogs, inject one 1 mL dose subcutaneously using aseptic technique. A second dose is given 2 to 4 weeks after the first vaccination. Annual revaccination is recommended.Infovax-id® System
The INFOVAX-ID® System provides a simple and effective method of recording pertinent information on the vaccines administered to animals in a veterinary practice.
For vaccines requiring reconstitution, remove label from both vials and affix both labels to the animal’s medical chart.
Using The Infovax-id® System
1. |
Grasp the lower right hand corner of the tab at the arrow marked “Peel Here” between your thumb and forefinger (figure 1). |
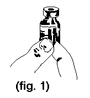 |
2. |
Pull steadily at a slight upward angle until the top portion of the label is separated from the vial (figure 2). |
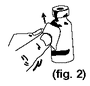 |
3. |
Place the label on the animal’s medical chart. Press down on the label to ensure adhesion. |
|
GiardiaVax Caution
Store in the dark at 2° to 7°C (35° to 45°F). AVOID FREEZING. SHAKE WELL. In case of anaphylactoid reaction, administer epinephrine.Thimerosal and gentamicin added as preservatives.
For Veterinary Use Only
© 2004 Fort Dodge Animal Health. All Rights Reserved.
GiardiaVax Caution
In the absence of a veterinarian-client-patient relationship, Federal law prohibits the relabeling, repackaging, resale, or redistribution of the individual contents of this package. (9 CFR Part 112.6)Fort Dodge Animal Health, Fort Dodge, Iowa 50501 Usa
U.S. Vet. License No. 112
|
25 DOSES |
25 - 1 mL Vials of Vaccine |
8924 01104 2795D |
Nac No.
10030926Division of Wyeth
235 E. 42ND ST., NEW YORK, NY, 10017
| Telephone: | 269-833-4000 | |
| Customer Service: | 800-733-5500 and 800-793-0596 | |
| Veterinary Medical Investigations & Product Support: | 800-366-5288 | |
| Technical Services (USA): | 800-366-5288 | |
| Website: | http://pfizerah.com |
 |
Every effort has been made to ensure the accuracy of the GiardiaVax information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
