EVICTO selamectin topical solution (45 mg) (Canada)
This treatment applies to the following species: Company: Virbac
Company: Virbac
Veterinary Use Only
Topical Parasiticide for Dogs and Cats
DIN 02495260, DIN 02495279, DIN 02495287, DIN 02495295, DIN 02495309, DIN 02495317, DIN 02495325, DIN 02495333
Active Ingredient
Selamectin is a semi-synthetic compound of the avermectin class.
PRODUCT DESCRIPTION:
Evicto topical solution is available as a colourless to yellow, ready to use solution in single dose tubes for topical (dermal) treatment of dogs and cats not less than six weeks of age. The content of each tube is formulated to provide a minimum of 6 mg/kg of body weight of selamectin.
EVICTO selamectin topical solution (45 mg) Indications
DOGS: For the treatment and control of flea (Ctenocephalides canis, C. felis), ear mite (Otodectes cynotis), sarcoptic mange mite (Sarcoptes scabiei) and tick (Rhipicephalus sanguineus) infestations, and for the prevention of heartworm disease caused by Dirofilaria immitis. Evicto topical solution is also indicated as an aid in the treatment and control of roundworm (Toxocara canis) infections and tick (Dermacentor variabilis) infestations.
CATS: For the treatment and control of flea (Ctenocephalides canis, C. felis) and ear mite (Otodectes cynotis) infestations, intestinal hookworm (Ancylostoma tubaeforme) and roundworm (Toxocara cati) infections, and for the prevention of heartworm disease caused by Dirofilaria immitis.
DOSAGE:
Evicto topical solution is applied topically to the skin at the recommended minimum dose of 6 mg selamectin per kg of body weight, once a month. Administer Evicto topical solution in accordance with the following tables:
|
Cats |
Mg per tube |
Potency (mg/mL) |
Administered volume (nominal tube sizes - mL) |
|
≤ 2.5 |
15 mg |
60 |
0.25 ml |
|
2.6 - 7.5 |
45 mg |
60 |
0.75 ml |
|
7.6 - 10.0 |
60 mg |
60 |
1.0 ml |
|
> 10.0 |
Appropriate combination of tubes |
60 |
Appropriate combination of tubes |
|
Dogs |
Mg per tube |
Potency (mg/mL) |
Administered volume (nominal tube sizes - mL) |
|
≤ 2.5 |
15 mg |
60 |
0.25 |
|
2.6 - 5.0 |
30 mg |
120 |
0.25 |
|
5.1 - 10.0 |
60 mg |
120 |
0.5 |
|
10.1 - 20.0 |
120 mg |
120 |
1.0 |
|
20.1 - 40.0 |
240 mg |
120 |
2.0 |
|
40.1 - 60.0 |
360 mg |
120 |
3.0 |
|
> 60 |
Appropriate combination of tubes |
120 |
Appropriate combination of tubes |
ADMINISTRATION:
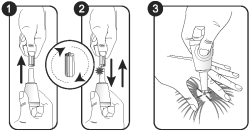
To facilitate application of Evicto, follow these basic steps. Evicto should be applied when the pet’s haircoat is dry. However, bathing or immersing your pet in water 2 hours after application will not reduce the effectiveness of this treatment.
Remove the Evicto tube from its protective package
1. Holding the tube upright, remove the cap.
2. Invert the cap and place other end back onto applicator tip. Push the cap down to break the applicator seal. Remove the cap prior to treatment application
3. Part the hair on the back of the animal at the base of the neck, in front of the shoulder blades, until the skin is visible.
Apply the tip of the Evicto tube directly to the skin without massaging. Squeeze the tube firmly to empty the contents in one spot. Avoid contact between Evicto and your fingers.
Discard empty tubes in your ordinary household refuse.
Contraindications
Do not use in sick, debilitated or underweight animals.
CAUTIONS:
Evicto topical solution may be safely administered to heartworm infected dogs and cats, however, it is recommended, in accordance with good veterinary practice, that all dogs ≥ 6 months of age be tested for existing heartworm infections before beginning medication with Evicto topical solution. Cats ≥ 6 months of age in heartworm endemic areas may also be tested to determine the presence of existing heartworm infections before beginning medication with Evicto topical solution.
Evicto topical solution is not effective against adult D. immitis; however it may decrease the number of circulating microfilariae.
Evicto topical solution should be used in animals of six or more weeks of age.
Warnings
Keep out of reach of children.
May be irritating to skin and eyes. Wash hands after use and wash off any product in contact with the skin immediately with soap and water. If contact with eyes occurs, then flush eyes copiously with water. In case of ingestion by a human, contact a physician immediately.
Adverse Reactions
The following adverse events are based on voluntary post-approval global adverse drug experience reporting (excluding European countries). Not all adverse events are reported. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data.
The following adverse events are listed in decreasing order of reporting frequency:
● CATS: application site hair change, lack of expected efficacy, lethargy, anorexia, emesis, application site pruritus or lesion or erythema, diarrhea, ataxia and death. Product causality could not be confirmed in the majority of cases with fatal outcome.
● DOGS: lack of expected efficacy, emesis, lethargy, diarrhea, anorexia, pruritus, convulsion, application site hair change, ataxia and muscle tremor. As with other macrocytic lactones, the reversible neurological signs listed have been very rarely observed after the use of selamectin.
In the majority of lack of expected efficacy reports the product causality could not be confirmed. adherence to the product’s dosage, treatment schedule and duration as well as consideration of specific parasite biology are of utmost importance in insuring success in parasite treatment and/or prevention.
Collies and other herding breeds are known to be more sensitive to the macrocyclic lactone class of medications. Signs of toxicity that can be seen with overdose include: depression, hypersalivation, tremor and ataxia.
Although adverse reactions may occur with use of any pharmaceutical product, it is recommended that any sign of new illness observed during the treatment with Evicto is thoroughly investigated.
EFFICACY:
Flea Control in Dogs and Cats:
If the dog or cat is already infested with fleas when the first dose of Evicto topical solution is administered, then adult fleas on the animal are killed and no viable flea eggs are produced after the first administration. This stops flea reproduction. An environmental infestation of fleas may persist for a short time after beginning treatment with Evicto topical solution because of the emergence of adult fleas from pupae. However, large reductions in flea infestations are observable after just one monthly treatment as flea larvae in the animal surroundings are killed or prevented from developing to adults by treatment with Evicto topical solution.
For the lasting control of flea infestations, Evicto topical solution should be administered at monthly intervals throughout the flea season, starting one month before fleas become active. This ensures that fleas infesting the animal are killed and that no viable flea eggs are produced by these fleas. This breaks the flea life cycle and controls flea infestations. Results of clinical field efficacy studies using selamectin topical solution monthly demonstrated control of flea infestations and improved the clinical manifestation of flea infestations, including flea allergy dermatitis.
Heartworm Prevention in Dogs and Cats:
For the prevention of heartworm disease, Evicto topical solution must be administered on a monthly basis. Evicto topical solution may be administered year-round or at least within one month after the animal’s first exposure to mosquitoes and monthly thereafter until the end of the mosquito season. The final dose must be given within one month after the last exposure to mosquitoes. If a dose is missed and a monthly interval between dosing is exceeded then immediate administration of Evicto topical solution and resumption of monthly dosing will minimize the opportunity for the development of adult heartworms. When replacing another heartworm preventive product in a heartworm disease prevention program, the first dose of Evicto topical solution must be given within a month of the last dose of the former medication.
Ear Mite Treatment in Dogs and Cats:
For the elimination of ear mites (O. cynotis) in dogs and cats, Evicto topical solution should be administered once as a single dose. A second monthly dose of Evicto topical solution may be required to eliminate mites in some dogs. Monthly use of Evicto topical solution will treat any subsequent ear mite infestations. Many animals treated for otoacariasis have concurrent otitis externa involving secondary yeast or bacterial infections. O. cynotis causes primary inflammation and pruritus of the external ear canal and predisposes to these secondary infections. Therefore, cats and dogs with otoacariasis complicated by secondary yeast and/or bacterial infections may require supportive therapy in addition to treatment with Evicto topical solution. Cleansing of the infested ears is recommended to remove the debris.
Nematode Treatment in Dogs and Cats:
For the treatment and control of intestinal hookworm (A. tubaeforme) and roundworm (T. cati) infections in cats, Evicto topical solution should be applied once as a single treatment, and monthly thereafter to control infections.
As an aid in the treatment of intestinal roundworm (T. canis) infections in dogs, administer two monthly treatments, and monthly thereafter to control infections.
Sarcoptic Mange Treatment in Dogs:
A single dose of Evicto topical solution is safe and highly efficacious against natural infestations of S. scabiei in dogs, however, for complete eradication two doses may be required. Monthly use of Evicto topical solution will treat any subsequent Sarcoptes mite infestations.
Tick Treatment in Dogs:
For tick (Rhipicephalus sanguineus, Dermacentor variabilis) infestations, Evicto topical solution should be administered on a monthly basis. In heavy tick infestations, adequate efficacy may not be achieved after the first dose. In these cases, one additional dose may be administered two weeks after the previous dose, with monthly dosing continued thereafter.
ANIMAL SAFETY:
DOGS:
Selamectin topical solution has been tested with no adverse reactions in dogs of over 100 different pure and mixed breeds including Collies, and in pregnant and lactating females, breeding males and females, and puppies six weeks of age and older. Selamectin topical solution was administered at 10 times the recommended dose, and no adverse effects were observed. Selamectin topical solution was also administered at 3 times the recommended dose to heartworm infected dogs, and no adverse effects were observed.
CATS:
Selamectin topical solution has been tested with no serious adverse effects in cats of over 15 different pure and mixed breeds and in pregnant and lactating females, breeding males and females and kittens six weeks of age and older. Less than 1% of cats treated in the field studies had a transient alopecia at or near the site of application, possibly due to self grooming. In safety studies, selamectin topical solution was applied at 10 times the recommended dose, and no adverse effects were noted. Selamectin topical solution was also applied at 4 times the recommended dose to heartworm infected cats, and no adverse effects were observed. Accidental oral administration of Evicto topical solution to cats may induce salivation or vomiting. In well-controlled clinical studies, selamectin topical solution was used safely in animals receiving other frequently used veterinary products such as vaccines, antiparasitics, antibiotics, steroids, pesticides and shampoos.
STORAGE CONDITIONS:
Store between 15°C and 30°C. Protect from light. Keep in the original carton until ready for use. Flammable - Keep away from heat, sparks, open flames or other sources of ignition.
PRESENTATION:
Available in the following strengths of 15, 45 and 60 mg for cats and 15, 30, 60, 120, 240 and 360 mg for dogs, in pack size of 6 tubes. Not all pack sizes may be marketed.
Virbac AH, Inc., P.O. Box 162059, Fort Worth, TX 76161 USA
Imported and distributed by: Virbac Canada Inc., 231-209 Shearson Crescent, Cambridge, ON, N1T 1J5
84127001
CPN: 1177105.0
209-231 SHEARSON CRESCENT, CAMBRIDGE, ON, N1T 1J5
| Toll-Free: | 866-458-3350 | |
| Fax: | 844-458-4004 | |
| Website: | https://ca.virbac.com/ |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
