Draxxin KP (Canada)
This treatment applies to the following species: Company: Zoetis
Company: Zoetis
tulathromycin and ketoprofen injection
Antibiotic and non-steroidal anti-inflammatory for cattle
Sterile
DIN 02516683
Veterinary Use Only
Description
DRAXXIN® KP injection is a ready to use sterile parenteral preparation containing tulathromycin, a semi-synthetic macrolide antibiotic of the subclass triamilide, and ketoprofen, a non-steroidal anti-inflammatory drug (NSAID). Each mL of DRAXXIN KP contains 100 mg of tulathromycin as a free base and 120 mg ketoprofen as a free acid in a 50% propylene glycol vehicle, monothioglycerol (5 mg/mL), 2-pyrrolidone (70 mg/mL), citric acid (20 mg/mL) and sodium hydroxide/hydrochloric acid added to adjust pH.
DRAXXIN KP contains an equilibrated mixture of two isomeric forms of tulathromycin in a 9:1 ratio and a racemic mixture of ketoprofen. The structures of the tulathromycin isomers and ketoprofen are shown below:
Figure 1. Tulathromycin structures

The chemical names of the isomers are (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R) - 13 - [[2,6 - dideoxy - 3 - C - methyl - 3 - O - methyl - 4 - C - [(propylamino)methyl] - α - L - ribo - hexopyranosyl]oxy] - 2 - ethyl - 3,4,10 - trihydroxy - 3,5,8,10,12,14 - hexamethyl - 11 - [[3,4,6 - trideoxy - 3 - (dimethylamino) - β - D - xylo - hexopyranosyl] - oxy] - 1 - oxa - 6 - azacyclopentadecan - 15 - one and (2R,3R,6R,8R,9R,10S,11S,12R) - 11 - [[2,6 - dideoxy - 3 - C - methyl - 3 - O - methyl - 4 - C - [(propylamino)methyl] - α - L - ribo - hexopyranosyl]oxy] - 2 - [(1S,2R) - 1,2 - dihydroxy - 1 - methylbutyl] - 8 - hydroxy - 3,6,8,10,12 - pentamethyl - 9 - [[3,4,6 - trideoxy - 3 - (dimethylamino) - β - D - xylo - hexopyranosyl]oxy] - 1 - oxa - 4 - azacyclotridecan - 13 - one, respectively.
Figure 2. Ketoprofen Structure
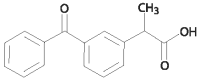
The chemical name of ketoprofen is 2-(3-Benzoylphenyl) propanoic acid.
Draxxin KP Indications
DRAXXIN KP is indicated for the treatment of clinical bovine respiratory disease (BRD), with accompanying pyrexia, associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis susceptible to tulathromycin in beef and non-lactating dairy cattle 2 months of age and older.
Draxxin KP Dosage And Administration
Inject subcutaneously as a single dose in the neck at a dosage of 2.5 mg tulathromycin and 3.0 mg ketoprofen/kg (1.25 mL/50 kg) bodyweight (BW). Do not inject more than 10 mL per injection site.
Table 1. DRAXXIN KP Dosing Guide
|
Animal Weight (kg) |
Dose Volume (mL) |
|
50 |
1.25 |
|
100 |
2.5 |
|
200 |
5.0 |
|
300 |
7.5 |
|
400 |
10.0 |
|
500 |
12.5 |
|
600 |
15.0 |
Contraindications
The use of DRAXXIN KP is contraindicated in animals previously found to be hypersensitive to tulathromycin or ketoprofen.
Draxxin KP Cautions
Not for use in reproducing animals over one year of age because reproductive safety testing has not been conducted. The effects of DRAXXIN KP on bovine reproductive performance, pregnancy, and lactation have not been determined.
Not for use in calves under 2 months of age.
Administration of DRAXXIN KP may result in injection site swelling that appears the day after treatment and may persist for at least 32 days post-injection. This may result in trim loss of edible tissue at slaughter.
As a class, cyclo-oxygenase inhibitory NSAIDs (e.g., ketoprofen) may be associated with gastrointestinal, hepatic and renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual animal. Cattle at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction. Use judiciously when renal impairment or gastric ulceration is suspected. Since many NSAIDs possess the potential to induce gastrointestinal ulceration, concomitant use of DRAXXIN KP with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided or closely monitored. Discontinue use if fecal blood is observed.
Use of DRAXXIN KP should consider the susceptibility of BRD pathogens to tulathromycin because of reported resistance in Mannheimia haemolytica and Pasteurella multocida from beef cattle in some regions of Canada. Inappropriate use of DRAXXIN KP may increase the prevalence of bacteria resistant to tulathromycin and other macrolide antibiotics and may decrease their effectiveness.
Warnings
Treated cattle must not be slaughtered for use in food for at least 49 days after the latest treatment with this drug. Do not use in dairy cows 20 months of age and older. Do not use in calves to be processed for veal. The withdrawal period has not been established in pre-ruminating calves.
Caution should be taken to avoid accidental self-injection and contact with eyes or skin. In case of accidental self-injection, seek medical advice immediately and show the label to the physician. In case of eye exposure, flush with water. In case of skin exposure, wash with soap and water.
To limit the development of antimicrobial resistance, the choice of this drug as the most appropriate treatment should be confirmed by clinical experience supported, where possible, by pathogen culture and drug susceptibility testing.
Keep out of reach of children.
Adverse Reactions
Administration of NSAIDs can result in gastric or renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual animal. Cattle at greatest risk for toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with pre-existing gastric ulcers, renal, cardiovascular, and/or hepatic dysfunction.
On rare occurrences, anaphylactic type reactions, sometimes fatal, have been reported with the use of this product.
Clinical Pharmacology
Mode of Action
Ketoprofen is a propionic acid derivate and NSAID with anti-inflammatory, analgesic and antipyretic effects. Ketoprofen inhibits the activity of the enzymes cyclo-oxygenase I and II, resulting in a decreased formation of precursors of prostaglandins and thromboxanes. Ketoprofen also causes a decrease in the formation of thromboxane A2 synthesis by thromboxane synthase, thereby inhibiting platelet aggregation.
The principal mechanism of action of tulathromycin against bacteria involves direct inhibition of essential protein biosynthesis by selective binding to bacterial 50S ribosomal subunits. Tulathromycin acts by stimulating the dissociation of peptidyl-tRNA from the ribosome during the translocation process.
Clinical Pharmacology
In a Good Laboratory Practice-compliant pharmacokinetic study, 60 cattle received one of 3 treatments: 2.5 mg tulathromycin/kg BW, 3.0 mg ketoprofen/kg BW or a combination of the two active ingredients (2.5 mg tulathromycin and 3.0 mg of ketoprofen/kg BW) via subcutaneous injection. Blood samples were obtained prior to treatment administration and at 20 min, 40 min, 1, 1.5, 2, 3, 4, 6, 10, 24, 28, 32, 48, 52, 56, 72, 120, 168, 216, 264, 336, and 360 hours after treatment administration. The samples were analyzed using validated high-performance liquid chromatography-mass spectrometry (LC-MS/MS) to measure tulathromycin and ketoprofen concentrations. The tulathromycin/ketoprofen combination treatment had a lower ketoprofen peak concentration (Cmax) than the ketoprofen treatment alone, at 2,060 ng/mL compared to 6,310 ng/mL (ratio = 0.33, 90% CI: 0.27-0.40). However, the AUC0-t(last) for ketoprofen was higher in the combined ketoprofen/tulathromycin group at 26,200 ng•h/mL compared to 22,600 ng•h/mL in the ketoprofen monotherapy group (ratio = 1.16, 90% CI: 1.07-1.26). The lower Cmax but higher AUC0-t(last) for ketoprofen in the combination product likely resulted from a longer terminal half-life (t1/2) and a longer time to reach the maximum concentration (tmax) compared to the ketoprofen monotherapy (t1/2: 6.78 and 2.72 hours, respectively; tmax: 4.00 and 0.83 hours, respectively). This indicates a potential interaction between tulathromycin and ketoprofen, altering the absorption of ketoprofen. Tulathromycin pharmacokinetics indicated that the combination treatment was equally bioavailable when compared to tulathromycin as a monotherapy with a mean AUC0-t(last) of 13,400 and 12,800 ng•h/mL, respectively (ratio = 1.04, 90% CI: 0.95-1.14). The Cmax was lower for tulathromycin in the combination treatment than tulathromycin as a monotherapy at 413 and 590 ng/mL, respectively (ratio = 0.70, 90% CI: 0.51-0.96). However, by 6 hours after treatment administration, the AUC0-6h of tulathromycin in the combination treatment and monotherapy was similar (1,570 and 1,770 ng•h/mL, respectively; ratio = 0.89, 90% CI: 0.76-1.05).
Microbiology
Based on data provided for DRAXXIN®, tulathromycin has demonstrated in vitro activity against Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis, four pathogens associated with BRD. In Canada, literature data have shown the emergence of resistance to tulathromycin (with minimal inhibitory concentrations of ≥64 µg/mL) in BRD pathogens.
Efficacy
A multi-location US field study was conducted to evaluate the effectiveness of DRAXXIN KP for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis, and the reduction of its associated pyrexia. A total of 819 cattle with clinical signs of BRD - moderate to severe depression and respiratory distress, and a rectal temperature ≥40°C (104.5°F) - were enrolled and randomly allocated to be treated with either saline (0.025 mL/kg BW), tulathromycin (2.5 mg/kg BW) or DRAXXIN KP (2.5 mg tulathromycin/kg BW and 3 mg ketoprofen/kg BW) administered once by subcutaneous injection. Rectal temperatures were measured six hours after treatment administration, and animals were observed for clinical signs of BRD through 14 days post-treatment. An animal was classified as a treatment success for the treatment of BRD if it was not classified as a failure prior to Day 14 and had normal or mild depression and respiratory distress scores and a rectal temperature <40°C (104.5°F) on Day 14. An animal was classified as a treatment success for the reduction of pyrexia associated with BRD if it displayed ≥1°C (2°F) reduction in rectal temperature at 6 hours post-treatment compared to pre-treatment. A sufficient number of Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis were isolated from cattle in the study to demonstrate that these BRD pathogens were contributing to the observed disease. The DRAXXIN KP-treated group had a significantly higher success rate for the treatment of BRD compared to the saline-treated group (76.2±3.6% and 31.6±4.1%, respectively, P=0.002), and the success rate in the DRAXXIN KP-treated group was statistically non-inferior (P=0.30) to that in the group receiving tulathromycin alone (71.9±3.4%). The success rate for the reduction of pyrexia associated with BRD in the DRAXXIN KP-treated group was significantly higher than the saline-treated group (83.8±7.0% and 2.4±1.5%, respectively, P=0.001) and the tulathromycin-treated group (83.9±6.6% and 5.4±2.7%, respectively, P=0.003) at 6 hours post-treatment.
Animal Safety
Thirty-two (16 male and 16 female) growing cattle were enrolled in a margin of safety study. Calves were randomly allocated to be injected subcutaneously with either saline, DRAXXIN KP at the label dose of 2.5 mg tulathromycin/3.0 mg ketoprofen/kg BW (1X), DRAXXIN KP at 3 times the label dose (3X), or DRAXXIN KP at 5 times the label dose (5X). The calves received three doses at 14-day intervals. Daily clinical observations were conducted by a veterinarian, as well as, daily general health observations and injection site evaluations. Samples were collected for urinalysis, fecal occult blood, hematology, serum chemistry and coagulation and for pharmacokinetics. At the conclusion of the study, all animals were euthanized and necropsied for gross pathology and a histopathological evaluation. Injection site lesion volumes for the first injection site were calculated. Injection site reactions were noted in all DRAXXIN KP-treated animals, and the size and incidence of injection site lesions were greater for all treatment groups compared to the saline (control) group. Swelling and hardness of the injection site persisted until the end of the study for the majority of animals in all treatment groups.
Test article-related differences in serum chemistry parameters consisted of lower alkaline phosphatase in the 5X group; lower albumin in the 3X and 5X groups; lower total protein (TP) and serum calcium in the 1X, 3X and 5X groups; and higher creatine kinase in the 1X, 3X, and 5X groups. The changes for serum calcium and albumin were considered clinically insignificant because all values were within the normal reference range, and the changes for TP were considered secondary to the differences in albumin. In addition, test article-related increases in absolute and segmented neutrophils for all treatment groups were observed when compared to the control group. The changes observed in serum chemistry and hematology were likely associated with transient inflammation and tissue reaction at the injection site, and are likely of a low clinical significance. No test article-related changes were detected in parameters related to coagulation or in the results of urinalysis. Ketoprofen plasma concentrations were below the limit of detection prior to the second and third doses, while there was a modest accumulation of tulathromycin over the 14-day dosing interval. There was a dose proportional increase in tulathromycin AUC0-t(last) with an increase in dose, while there was slightly greater than dose proportional increase in ketoprofen AUC0-t(last). A single calf in the 5X group had test article-associated positive fecal occult blood samples on Days 15 and 29 of the study. Microscopic mucosal erosions of the pylorus of the abomasum were present in the 3X (n=2/8) and 5X (n=1/8) groups and was considered test article-related. Renal interstitial inflammation and minimal multifocal degeneration and regeneration occurred at similar incidence and severity in the treated and control calves. Subcutaneous injection of cattle with tulathromycin-ketoprofen at the label dose was well tolerated.
Storage
Store between 15 and 30°C.
PRESENTATION
DRAXXIN KP is available in 100 mL and 500 mL vials.
Zoetis® and Draxxin are registered trademarks of Zoetis or its licensors.
Zoetis Canada Inc., Kirkland QC H9H 4M7
10022339-11-0
CPN: 1198578.0
16,740 TRANS-CANADA HIGHWAY, KIRKLAND, QC, H9H 4M7
| Order Desk: | 800-663-8888 | |
| Technical Services Canada: | 800-461-0917 | |
| Technical Services USA: | 800-366-5288 | |
| Website: | www.zoetis.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
