CORTOTIC Hydrocortisone Aceponate Otic Spray (Canada)
This treatment applies to the following species: Company: Virbac
Company: Virbac
Anti-inflammatory for use in dogs
Veterinary Use Only
DIN 02545454
Description
CORTOTIC™ Otic Spray ear spray solution contains 0.584 mg/mL hydrocortisone aceponate.
CORTOTIC Hydrocortisone Aceponate Otic Spray Indications
For the treatment of clinical signs associated with acute erythroceruminous otitis externa.
Directions For Use
The recommended dosage is 0.44 mL of CORTOTIC™ Otic Spray per affected ear once a day for 7 consecutive days. This dose is delivered by two pump activations. If the clinical signs of otitis are not resolved within 7 days, treatment may be extended for another 7 days, for a maximum of 14 days of treatment. Following treatment the ears should be re-evaluated. If there is an inadequate response, the diagnosis and treatment should be re-assessed.
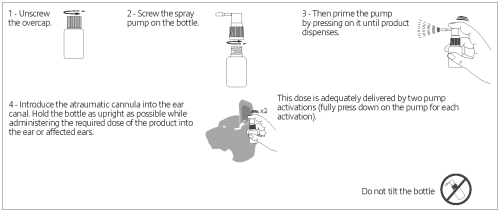
Instructions for use:
● Clean and dry the external ear canal with an otic cleanser before the first treatment.
● Do not repeat ear cleaning before further applications.
● Before first administration, remove the overcap and screw the spray pump on the bottle.
● Then activate the pump by pressing it down until the product is released.
● Introduce the atraumatic cannula into the ear canal and apply the product with two pump activations. Hold the bottle upright while administering the product in the affected ear(s). Special care should be taken to prevent exposure to the dog’s eyes.
● Massage the ear and auditory canal gently, but thoroughly, after each application to ensure proper distribution.
● Keep the spray pump screwed onto the bottle after use.
● If the spray pump has not been used for a long time, activate it once before applying the spray again.
Contraindications
Do not use in cases of hypersensitivity to the drug, to other corticosteroids or to the excipient, propylene glycol methyl ether. Do not use in animals with a perforated tympanic membrane. Do not use on dogs with ulcerated ear canals.
CAUTIONS: Before CORTOTIC™ Otic Spray is applied, the external auditory canal must be examined thoroughly to ensure that the eardrum is not perforated in order to prevent damage to the cochlear and vestibular apparatus.
Avoid contact with the dog’s eyes by restraining the dog’s head to prevent shaking. In case of accidental contact, rinse eyes thoroughly with water.
Safety has not been assessed in dogs under 4 months of age or weighing less than 4.8 kg.
Use of this product for longer than 14 days can suppress the Hypothalamic Pituitary Adrenal (HPA) axis. Local effects such as ototoxicity and deafness have not been evaluated after prolonged overexposure (see Target Animal Safety section). Do not exceed the recommended dose or duration of treatment.
Otitis externa is a multifactorial disease, where bacterial and yeast infection is often secondary in nature. The underlying dermatological condition should be identified and treated, in conjunction with treatment for any secondary infections/infestations. Additional diagnostics, including culture, should be performed if there is inadequate response to treatment. For parasitic otitis, appropriate acaricidal treatment should be implemented. The product has not been assessed in dogs with suppurative otitis externa.
Safety has not been established in breeding, pregnant or lactating dogs. Safety has not been established in dogs with impaired hepatic
or renal function.
The solvent in this product may stain certain materials including painted, varnished, or other household surfaces or furnishings.
Warnings
Keep out of reach of children.Eye irritant. Avoid contact with eyes including hand to eye contacts. In case of accidental eye contact, rinse with abundant quantities of water. In case of eye irritation, seek medical advice immediately and show the package insert or the label to the physician.
This active substance is potentially pharmacologically active at high doses of exposure. Avoid skin contact and wash hands after use. Replace the bottle in the outer carton and store in a safe place and out of the sight of children. In case of accidental skin contact, it is recommended to wash thoroughly with water.
In case of accidental ingestion, particularly by children, seek medical advice immediately and show the product insert or the label to the physician. FLAMMABLE. Do not spray directly on a flame or any incandescent material. Do not smoke while handling the veterinary medicinal product.
Adverse Reactions
In a single-blinded, randomized, controlled European field study, 97 client-owned dogs received CORTOTIC™ Otic Spray and 104 dogs received a positive control drug containing (miconazole nitrate, prednisolone acetate, polymyxin B sulfate) to treat acute erythroceruminous otitis externa. Study dogs were 8.4 months to 14 years of age, weighed 2.8-70kg, and were of various breeds. During this study, 22 adverse events were recorded, of which 21 were unlikely related to drug administration. Ten (10.3%) of the dogs in the CORTOTIC™ Otic Spray group experienced 14 adverse events while 6 dogs (5.8%) in the positive control group experienced 8 adverse events. In the CORTOTIC™ Otic Spray group, dermatitis and eczema (2.1%) and otitis (2.1%) were the most common adverse events recorded, while in the control group it was emesis (1.9%). In both groups, the frequency of all other adverse events was ≤1%. One dog with evidence of ear pathology prior to study start developed head tilt 7 days after starting the CORTOTIC™ Otic Spray treatment, however, it resolved by the end of the study.Information for animal owners:
The CORTOTIC™ Otic Spray product was not shown to adversely affect hearing in controlled clinical studies, however, the use of otic preparations has been associated with transient/permanent deafness or partial hearing loss in some animals. If signs of hearing loss are observed, administration should be stopped, and a veterinarian consulted.
Clinical Pharmacology
Hydrocortisone aceponate is (11β,17,21-Trihydroxy pregn-4-ene-3,20-dione 21-acetate 17-propionate) with the molecular formula: C26H36O7. Molecular mass: Mr 460.57.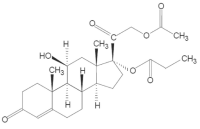
CORTOTIC™ Otic Spray contains the active substance hydrocortisone aceponate. Hydrocortisone aceponate (HCA) belongs to the diester class of the glucocorticosteroids with intrinsic glucocorticoid activity. Glucocorticoids reduce both inflammation and pruritus.
After auricular administration, HCA accumulates slightly and transiently within the dog’s skin, in the dermis and the hypodermis of the dog’s ear canal. HCA is transformed inside the skin structures resulting in high local potency. In laboratory animals, HCA is eliminated the same way as endogenous cortisol through urine and faeces.
TARGET ANIMAL SAFETY: In a blinded and controlled margin of safety study, 24 healthy Beagles of 4-5 months of age and weighing 4.8-7.9kg were treated with either 1X the CORTOTIC™ Otic Spray dose in both ears once daily for 42 consecutive days (3X recommended duration), 3X the CORTOTIC™ Otic Spray dose in both ears for 14 consecutive days (1X duration), or with an equal volume of sodium chloride 0.9% in both ears once daily for 42 consecutive days. All treatments were followed by a 63-day observation period. Study animals were evaluated for food consumption, weight, general health, vestibular function, and hearing (Clap Test), and had otoscopic examination, hematology/clinical chemistry/urinalysis and ACTH stimulation testing performed. No treatment related effects were noted on the dogs’ clinical health conditions, body weight, food consumption, or urinalysis tests. Some minor changes on hematology and clinical chemistry testing were consistent with the pharmacologic action of the drug: lymphopenia (in males), monocytosis (in males), neutrophilia (in males), increase in RBC indices (RBC count, hemoglobin, hematocrit), increased platelets (in males) and decreased blood urea nitrogen (in females). Changes were minor, reversible, non-adverse and remained within or slightly outside normal reference limits. Overdose treatment at either 1X the dose over 3X duration, or 3X the dose over 1X duration, induced a mild, reversible, pharmacological inhibition of the hypothalamic-pituitary-adrenal (HPA) axis and adrenal gland function (based on post-ACTH cortisol values) which resolved 29 to 57 days after treatment end, respectively. Transient lower cortisol levels during the treatment phase returned to basal levels at the end of the 63-day recovery observation period. In a controlled laboratory study, 16 healthy adult Beagle dogs were treated with 0.44mL of CORTOTIC™ Otic Spray or sodium chloride 0.9% in both ears once daily for 14 consecutive days. Study animals were examined, weighed and clinically observed throughout the study. Auditory Brainstem Response (ABR) testing and Adrenocorticotropic hormone (ACTH) stimulation testing were performed prior to treatment, at the end of treatment, and at the end of a 35-37 day observation period. After 14 consecutive days of treatment at the recommended therapeutic dose, CORTOTIC™ Otic Spray did not result in mortality or morbidity. No adverse effects were reported, except one dog had tympanic opacity (transient, white, opaque area in the tympanic membrane) which resolved during the observation period. There was no effect on body weight or food consumption parameters. No relevant ototoxic effects and no hearing impairment were observed. There was no effect on the HPA axis evaluations, and no induction of iatrogenic hypoadrenocorticism.
TARGET ANIMAL EFFICACY: In a single-blinded, randomized, controlled field study, 191 client-owned dogs (8.4 months to 14 years, 2.8-70kg) presenting with clinical signs of acute erythematous-ceruminous, non-parasitic otitis externa, with cytological evidence of the presence of bacteria or yeast were evaluated in a non-inferiority study comparing two treatments. Dogs were treated with either 2 pump sprays (0.44mL) of CORTOTIC™ Otic Spray once daily for 7-14 days (n=91; 42.3% treated for 7 days; 57.7% treated for 14 days) or 5 drops of positive control drug (miconazole nitrate, prednisone acetate, polymixin B sulfate) twice daily for 7-14 days (n=100; 42.7% treated for 7 days; 57.3% treated for 14 days). Study animals were evaluated for weight, general health, hearing (Clap Test), ear pain/pruritus, response to treatment, and had otoscopic examination, ear swab cytology/culture, and hematology/clinical chemistry/urinalysis testing performed. During the otoscopic examination, clinical signs including erythema, oedema/swelling, erosion/ulceration and discharge/exudate were assessed according to the Otitis Index Score (OTIS-3). Each clinical sign was given a score from 0 to 3 (e.g. 0=absent, 1=mild, 2=moderate, 3=severe) and the sum of all individual scores provided a Clinical Sum Score (CSS). Percentage of change in CSS at Day 28 was the primary efficacy endpoint. Non-inferiority of CORTOTIC™ Otic Spray versus the positive control drug was established since the percentage reduction in CSS was not significantly different (p=0.441) at 74.21 (+/-26.89) and 71.46 (+/-26.01), respectively. OTIS-3 scores of 3 or less were considered treatment successes. Treatment success was not significantly different between the groups on study day 42 (86% for CORTOTIC™ Otic Spray and 85% of the positive control group p=0.819). The relapse rate (until study day 42) after treatment was also not significantly different between the groups.
Storage
Store between 15 - 25°C. Keep in a dry place. Keep from freezing.Six month shelf life after opening.
PRESENTATION: High density polyethylene (HDPE) bottle of 20 mL filled with 16 mL of solution, closed with an HDPE screw overcap.
A spray pump is provided.
Package includes 1 bottle and 1 spray pump.
Virbac AH, Inc., PO Box 162059, Fort Worth, Texas, United States 76161
Imported and distributed in Canada by Virbac Canada, Inc., 231 Shearson Crescent, Suite 209, Cambridge, ON, Canada, N1T 1J5. 1-800-338-3659
CORTOTIC is a trademark of Virbac S.A.
© 2024 Virbac Corporation. All Rights Reserved.
11609
84345001
|
Net: |
|
|
16 mL |
84344901 84344801 |
CPN: 1177128.0
209-231 SHEARSON CRESCENT, CAMBRIDGE, ON, N1T 1J5
| Toll-Free: | 866-458-3350 | |
| Fax: | 844-458-4004 | |
| Website: | https://ca.virbac.com/ |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
