Cardalis 2.5 mg/20 mg chewable tablets (Canada)
This treatment applies to the following species: Company: Ceva Animal Health
Company: Ceva Animal Health
Benazepril hydrochloride and spironolactone chewable tablets
Veterinary Use Only
DIN 02542102
DIN 02542080
For Dogs
Description
CARDALIS® contains two active ingredients, benazepril hydrochloride and spironolactone, in a ratio of 1:8 respectively. CARDALIS® is supplied as brown oblong half-scored flavored chewable tablets.
Benazepril belongs to the angiotensin-converting enzyme (ACE) inhibitor class of drugs. Spironolactone, and its active metabolites, act as specific aldosterone receptor antagonists.
Both ACE inhibitors and aldosterone receptor antagonists act on the renin-angiotensin-aldosterone system.
Cardalis 2.5 mg/20 mg chewable tablets Indications
CARDALIS® is indicated with concurrent furosemide therapy for the management of clinical signs of mild, moderate, or severe congestive heart failure due to myxomatous mitral valve disease in dogs.
Directions For Use
CARDALIS® chewable tablets should be administered to the dog once a day at a dosage of 0.25 mg - 0.5 mg benazepril hydrochloride/kg bodyweight (b.w.) and 2 mg - 4 mg spironolactone/kg b.w., according to the following dosage table.
|
Bodyweight (kg) of dog |
Strength and number of tablets to be administered: |
|
|
CARDALIS® 2.5 mg/20 mg chewable tablets |
CARDALIS® 5 mg/40 mg chewable tablets |
|
|
2.5 - 5 |
1/2 |
|
|
> 5 - 10 |
1 |
|
|
> 10 - 20 |
|
1 |
The tablets should be administered with food, either mixed with a small amount of food offered to the dog just prior to the main meal, or with the meal itself.
Contraindications
Do not administer in conjunction with non-steroidal anti-inflammatory drugs in dogs with renal insufficiency.
Do not administer to dogs with hypoadrenocorticism (Addison’s Disease), hyperkalemia, or hyponatremia.
Do not administer to animals with known hypersensitivity to ACE inhibitors or spironolactone.
Cautions
CARDALIS® is only for use in dogs with clinical evidence of heart failure.
Renal function and serum potassium levels should be evaluated prior to initiating treatment with CARDALIS®. Regular monitoring of renal function and serum potassium levels is recommended as there may be an increased risk of hyperkalemia.
Use in dogs with renal insufficiency has not been evaluated. Dogs should be adequately hydrated to avoid renal toxicity.
Benazepril hydrochloride and spironolactone undergo extensive hepatic biotransformation. Care should be taken when using CARDALIS® in dogs with hepatic dysfunction.
Spironolactone decreases digoxin elimination and raises digoxin plasma concentrations. Dogs receiving digoxin and CARDALIS® should be closely monitored.
The safety of CARDALIS® has not been evaluated in growing dogs. Spironolactone has an antiandrogenic effect and should be used with caution in growing dogs. The safety of CARDALIS® has not been established in pregnant, lactating or breeding dogs.
Warnings
People with known hypersensitivity to benazepril or spironolactone should avoid contact with the product. Pregnant women should take special care to avoid accidental oral exposure because ACE inhibitors have been found to affect the unborn child during pregnancy in humans. Accidental ingestion, particularly by children, may lead to adverse events such as drowsiness, nausea and vomiting and diarrhea, and skin rashes. In case of accidental ingestion, seek medical advice immediately and show the package insert or the label to the physician. Wash hands after use. Keep out of reach of children.
Adverse Reactions
Although not all adverse reactions are reported, the following information is based on voluntary post-approval drug experience reporting. It is generally recognized that this results in significant under-reporting. The adverse events listed here reflect reporting and not necessarily causality. Adverse events are listed by body system, in decreasing order of frequency.
Systemic disorders: lethargy, anorexia, polydipsia
Digestive tract disorders: diarrhea, vomiting
Respiratory tract disorders: dyspnea, tachypnea, cough
Skin and appendage disorders: pruritus
Very rarely, death has been reported, resulting from clinical signs attributable to the progression of heart disease.
If you notice any serious adverse reactions or other adverse reactions not mentioned in the package insert, please inform your veterinarian.
A U.S.A. clinical field study comprised of a 360-day treatment period evaluated the safety and efficacy of CARDALIS® compared to benazepril hydrochloride in 569 client-owned dogs with left sided atrio-ventricular valvular insufficiency. The table below summarizes the adverse reactions not directly related to the progression of the disease that occurred in greater than 5% of the dogs treated with CARDALIS®.
|
Adverse Reactions Occurring in ≥ 5% of the CARDALIS® Treated Dogs |
||
|
Preferred Term |
CARDALIS® |
Benazepril hydrochloride |
|
Anorexia |
107 (38%) |
113 (40%) |
|
Vomiting |
70 (25%) |
51 (18%) |
|
Lethargy |
44 (16%) |
41 (14%) |
|
Diarrhea |
43 (15%) |
41 (14%) |
|
Renal insufficiency |
31 (11%) |
19 (6.7%) |
|
Collapse |
16 (5.6%) |
12 (4.2%) |
|
Hepatopathy |
16 (5.6%) |
8 (2.8%) |
The following adverse events were seen in fewer than 5% of the study animals, in decreasing order: urine abnormalities, fluid in abdomen, ataxia, weight loss, digestive tract disorder, hypertension, electrolyte disorder, bronchitis and hyperactivity.
The incidence of death, including euthanasia and sudden death, was similar in dogs treated with CARDALIS® or benazepril hydrochloride. In most cases, death was attributable to the progression of heart disease, or the clinical signs associated with congestive heart failure. Death of unknown cause were presumed to be cardiac in nature.
Serum magnesium and potassium values were significantly higher in the CARDALIS® group, although the mean values remained within the reference range and did not change significantly over time. These electrolyte changes are consistent with the potassium-sparing properties of spironolactone.
Other Scientific Information
Spironolactone:
Structural formula, including relative and absolute stereochemistry:
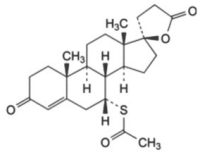
Molecular formula: C24H32O4S
Benazepril hydrochloride:
Structural formula, including relative and absolute stereochemistry:
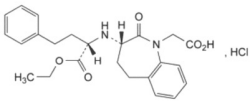
Molecular formula: C24H29ClN2O5
Clinical Pharmacology
Mechanism of Action: The main pharmacological target of spironolactone and benazepril is the renin-angiotensin-aldosterone system (RAAS) at different levels in the cascade. Spironolactone and its active metabolites (including 7-α-thiomethyl-spironolactone and canrenone) act as specific antagonists of aldosterone receptors by binding competitively to aldosterone receptors (also called mineralocorticoid receptors) located in the kidneys, heart and blood vessels.
Benazepril hydrochloride is a prodrug hydrolysed in vivo into its active metabolite, benazeprilat. Benazeprilat is a highly potent and selective inhibitor of angiotensin converting enzyme (ACE), thus preventing the conversion of inactive angiotensin I to active angiotensin II. This prevents deleterious effects of vasocontriction and aldosterone release, including sodium and water retention, and vascular and myocardial remodelling. However, aldosterone release is not fully controlled by ACE inhibitors because angiotensin II is also produced by non-ACE pathways (phenomenon known as “aldosterone breakthrough”). The aldosterone antagonist spironolactone and the ACE inhibitor benazepril hydrochloride inhibit both ACE and non-ACE pathways.
a) Pharmacokinetics
Spironolactone is rapidly and extensively metabolized in humans and experimental animals, with species differences in the metabolism and disposition. Spironolactone is metabolized by microsomal cytochrome P450 to a primary metabolite, canrenone, and a secondary metabolite, 7α-thiomethyl-spironolactone (TMS), which are used as markers for spironolactone in dog plasma. Systemic exposure to both metabolites was comparable when spironolactone was administered alone or in association with benazepril in dogs. Spironolactone absorption is affected by food and the dose should be administered consistently in fed conditions. Spironolactone is mainly excreted as metabolites. After oral administration of radiolabelled spironolactone to the dog, 70% of the dose is recovered in the feces and 20% in the urine.
After multiple oral doses of 40 mg of spironolactone with 5 mg of benazepril in dogs (n=24,) for 7 consecutive days, no accumulation was observed.
|
Mean Pharmacokinetic Parameters for Canrenone and TMS on Day 7 |
||
|
Parameters |
Canrenone (+SD) |
TMS (+SD) |
|
Tmax (hour) |
3.8 (+1.2) |
1.7 (+1.1) |
|
Cmax (μg/L) |
66.0 (+9.5) |
324.0 (+91.6) |
|
AUCo-t (h*μg/L) |
821.4 (+179.3) |
1866.0 (+660.4) |
Benazepril hydrochloride is a prodrug which, after absorption from the gastrointestinal tract, is rapidly converted in the liver to its active metabolite benazeprilat. Benazeprilat is itself poorly absorbed from the gastrointestinal tract and the overall bioavailability of benazeprilat after oral administration is estimated to be less than 7%.
After multiple oral doses of 5 mg of benazepril with 40 mg of spironolactone in dogs (n=24) for 7 consecutive days (steady state), no accumulation was observed. In dogs, benazeprilat is excreted approximately equally by both renal (45%) and hepatic (55%) routes.
|
Mean Pharmacokinetic Parameters for Benazeprilat on Day 7 |
|
|
Parameters |
Benazeprilat (+SD) |
|
Tmax (hour) |
1.4 (+1.1) |
|
Cmax (μg/L) |
52.4 (+16.3) |
|
AUCo-t (h*μg/L) |
168.5 (+29.1) |
Target Animal Safety
In a laboratory safety study, 32 healthy one-year old Beagle dogs (16 males and 16 females) were randomly assigned to an untreated control group or were dosed orally with CARDALIS® once daily for 26 weeks at 0, 1X, 3X, and 5X the maximum recommended daily dose (4 mg/kg spironolactone and 0.5 mg/kg benazepril hydrochloride). No dogs were removed and no unscheduled deaths occurred during the study. The dogs dosed with CARDALIS® showed no test article-related effects on food or water consumption, body weights, electrocardiographic findings, or blood pressure. The dogs in the 5X group had lower mean heart rates compared to the other groups at the end of the study. Increased mean serum potassium levels were found in dogs in all CARDALIS® dose groups but remained within reference range. There was a slight dose-related decrease in red cell mass (mean red blood cell count, hematocrit and hemoglobin) but all variables remained within reference ranges. There were decreases in mean prostate weights in the 3X and 5X groups and signs of slight to marked atrophy of prostate glandular tissue. There was a thickening of the zona glomerulosa of adrenal glands in both male and female in the 3X and 5X treated animals.
Systemic exposure to the active metabolites of spironolactone (canrenone and 7α-thiomethyl-spironolactone) and benazepril (benazeprilat) was shown at the three dose levels throughout the study, with no apparent sex effect. Canrenone and benazeprilat exposures were more than dose proportional at steady-state. There was no accumulation with repeated benazeprilat exposures; canrenone accumulation was 30%. Systemic exposure to 7-α-thiomethyl-spironolactone were variable by study day and dose of spironolactone; steady state, dose proportionality, and accumulation could not be determined.
Target Animal Efficacy
The efficacy of CARDALIS® was evaluated in a well-controlled U.S. multi-centre, blinded, randomized, 360-day field study in client-owned dogs. This study evaluated the efficacy of CARDALIS® in dogs diagnosed with congestive heart failure caused by left-sided atrio-ventricular valvular insufficiency (AVVI) compared to benazepril hydrochloride (active control).
Dogs ranged from 3 to 19 years of age and 2.3 to 70.5 kg at enrollment. The most common breeds were mixed breed, Cavalier King Charles Spaniel, chihuahua, shih tzu, Maltese, dachshund, and Yorkshire terrier. Enrolled dogs had radiographic evidence of congestive heart failure prior to enrollment or on Day 0 and exhibited clinical signs associated with left-sided AVVI, including exercise intolerance and/or dyspnea, echocardiographic evidence of left-atrial enlargement, moderate-to-severe mitral regurgitation, and presence of a left-sided cardiac murmur. Dogs with acquired heart disease other than left-sided AVVI, congenital heart defect, current positive heartworm antigen test, or syncope not related to heart disease, and dogs intended for breeding or known to be pregnant, or lactating were excluded.
A total of 569 dogs were treated with either CARDALIS® (284 dogs) at a minimum dose of 2 mg/kg spironolactone and 0.25 mg/kg benazepril hydrochloride once daily or benazepril hydrochloride alone (285 dogs) at a minimum dose of 0.25 mg/kg once daily. Doses were administered with food or within 30 minutes of feeding. All dogs received concurrent administration of oral furosemide throughout the study (up to 8 mg/kg) to manage pulmonary edema. Digoxin and calcium channel blockers were allowed for control of supraventricular arrhythmias. The use of injectable furosemide was permitted during the study evaluation period only if used in place of an equivalent oral dose.
The rate of treatment failure was the primary effectiveness variable used to compare CARDALIS® to benazepril hydrochloride alone. Treatment failure was defined as cardiac death or euthanasia (including death of unknown cause), recurrence or worsening or pulmonary edema, newly documented cardiogenic ascites, or clinical signs or congestive heart failure requiring administration of a furosemide dosage higher than 8 mg/kg/day. Failure rates at study days 30, 90, 180 and 270 were also evaluated as secondary outcomes.
The rate of failure in the CARDALIS® group estimated from the model analysis was statistically different (p=0.0433) and numerically lower than that of the benazepril hydrochloride alone group.
|
Treatment Failure Rates by Treatment Group (Least Square Means) |
||||
|
Group |
Number of Dogs |
Percent Treatment Failurea,b |
95% Confidence Interval |
p-value |
|
CARDALIS® |
216 |
80.59% |
72.57, 86.69% |
0.0433 |
|
Benazepril Hydrochloride |
198 |
88.09% |
81.40, 92.59% |
|
|
a= back-transformed from the logit transformation used in the statistical analysis which included random effects associated with study site and the site by treatment interaction b= calculated based on individual animal results |
||||
Further, the rate of failure in the CARDALIS® group was significantly lower than the group administered benazepril hydrochloride alone at all evaluation periods past study Day 30. The dogs in the CARDALIS® group exhibited a longer median time-to-failure when compared to the control group.
CARDALIS® was administered in dogs concurrently receiving furosemide, digoxin, calcium channel blockers, antiparasitic, analgesics/anti-inflammatories, antibacterials, routine canine vaccines, respiratory treatments, and gastrointestinal treatments.
Palatability
During the field study, 233 dogs were offered CARDALIS® once daily for 14 days. CARDALIS® was accepted voluntarily, with or without food, in 87.6% of the 3178 reported doses.
Storage
Store between 15 and 30°C. Upon opening, use within 6 months.
Presentation
CARDALIS® is supplied in two sizes: 2.5 mg benazepril hydrochloride and 20 mg spironolactone and 5 mg benazepril hydrochloride and 40 mg spironolactone in white plastic (HDPE) bottles with a child-resistant closure in a cardboard box.
Bottle sizes of 30 flavoured chewable tablets.
Ceva Animal Health Inc., 150 Research Lane, Suite 225, Guelph ON N1G 4T2
1-800-510-8864
CARDALIS® is a registered trademark of Ceva Santé Animale, France.
C6539-3120106
CPN: 1221169.0
150 RESEARCH LANE, SUITE 225, GUELPH, ON, N1G 4T2
| Telephone: | 519-650-9570 | |
| Toll-Free: | 800-510-8864 | |
| Fax: | 519-650-9576 | |
| Website: | www.ceva-canada.ca | |
| Email: | service.canada@ceva.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-03-02
