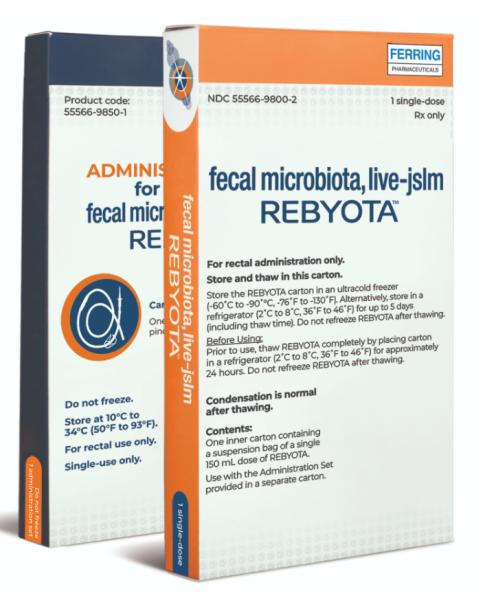
Rebyota
Generic name: (fecal microbiota, live-jslm)
Dosage form: suspension, for rectal use
Drug class: Miscellaneous uncategorized agents
What is Rebyota?
Rebyota is a fecal microbiota product used for the prevention of recurrence of Clostridioides difficile infection (CDI).
Rebyota is a fecal transplant product manufactured from human fecal matter donated by screened individuals. It works by facilitating the restoration of the gut flora to prevent further episodes of Clostridioides difficile infection.
Rebyota is administered rectally 24 to 72 hours after the last dose of antibiotics for the treatment of recurrent CDI.
Rebyota is not indicated for treatment of CDI.
Rebyota has not been studied in patients below 18 years of age.
What is Rebyota used to treat?
Rebyota is used for the prevention of recurrent Clostridioides difficile infection (CDI) in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI.
Clostridioides difficile infection is a bacterial infection of the gut that can be potentially life-threatening. It is caused by a change to the balance of microorganisms in the gut that allows the Clostridioides difficile bacteria to multiply and release harmful toxins. Symptoms of CDI include diarrhea, abdominal pain, fever, colitis (inflammation of the colon), and in some cases, organ failure and death.
After recovery from CDI, individuals may get the infection again multiple times, a condition known as recurrent CDI.
Important information
You should not receive Rebyota if you have a history of a severe allergic reaction to Rebyota or any of its components.
You should report to your doctor if you think you may have acquired any infection after administration. Rebyota is manufactured from human fecal matter and may carry a risk of transmitting infectious agents.
You should talk to your doctor about any known food allergies and be prepared should you experience any reaction. Rebyota is manufactured from human fecal matter and may contain food allergens.
You should not take any oral antibiotic therapy for up to 8 weeks after receiving Rebyota.
Before you receive Rebyota
Before you receive Rebyota, tell your doctor if you have any food allergies. Rebyota is manufactured from human fecal matter and may contain food allergens, although the potential for the product to cause adverse reactions due to food allergens is unknown.
Appropriate medical treatment must be immediately available in case you experience an allergic reaction.
What other drugs will affect Rebyota?
You should not take any oral antibiotic therapy for up to 8 weeks after receiving Rebyota unless directed by your doctor.
How will I receive Rebyota?
Rebyota is administered into the rectum by your health care provider.
You will be asked to empty your bladder and bowel before the procedure, if possible.
Your health care provider will position you on your left side, or in the knee-chest position with your buttocks elevated.
A water-soluble lubricant is inserted into the rectum before receiving Rebyota to prevent any discomfort.
The administration tube attached to the Rebyota bag is then inserted into the rectum and the bag is raised to allow the contents to be delivered by gravity. The bag should not be squeezed.
When the entire contents of the bag have been delivered into the rectum, the administration tube is withdrawn.
You will be asked to remain in the same position (left side or knee-chest) for up to 15 minutes to minimize any cramping that may occur.
Dosing information
Usual Adult Dose for Prevention of Recurrent Clostridioides difficile Infection:
150 mL administered rectally as a single dose
Use: prevention of recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI.
Rebyota side effects
Common side effects may include:
- stomach pain
- diarrhea,
- bloating,
- gas, and
- nausea.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.FDA.gov/medwatch or call 1-800-332-1088.
Related/similar drugs
Vowst
Vowst capsules are used to prevent the recurrence of Clostridioides difficile infection (CDI) after ...
Zinplava
Zinplava (bezlotoxumab) is used to prevent Clostridium difficile infection recurrence. Zinplava ...
Ozempic
Learn about Ozempic (semaglutide) for type 2 diabetes treatment, weight management, cardiovascular ...
Accutane
Accutane (isotretinoin) is a form of vitamin A and is used to treat severe nodular acne. Includes ...
Adakveo
Adakveo (crizanlizumab-tmca) is used for the prevention of vasoocclusive crises (VOCs) in patients ...
Ammonul
Ammonul is used to treat a condition caused by too much ammonia in the blood. Includes Ammonul side ...
Amnesteem
Amnesteem is used for acne, acute nonlymphocytic leukemia, granuloma annulare, rosacea
Amondys 45
Amondys 45 (casimersen) is used to treat Duchenne muscular dystrophy (DMD) with a genetic mutation ...
Storage of Rebyota
Rebyota should be stored in an ultracold freezer (-60˚C to -90˚C or -76˚F to -130˚F). Alternatively, store in a refrigerator (2˚C to 8˚C or 36˚F to 46˚F) for up to 5 days (including thaw time). Do not refreeze Rebyota after thawing.
Before using, Rebyota must be thawed completely in a refrigerator (2˚C to 8˚C or 36˚F to 46˚F) for approximately 24 hours.
Rebyota Biosimilars
Biosimilar and interchangeable products are biological products that are highly similar to and have no clinically meaningful differences from the reference product.
Reference products
These are biological products that have already been approved by the FDA, against which biosimilar products are compared. There is 1 for Rebyota.
Rebyota (fecal microbiota, live-jslm) - Ferring Pharmaceuticals Inc.
| Formulation type | Strength |
|---|---|
| Single-Use Bottle | BETWEEN 1 X10^8 AND 5 X10^10 CFU |
References
More about Rebyota (fecal microbiota, live)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous uncategorized agents
Professional resources
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.