Ziihera: Package Insert / Prescribing Info
Package insert / product label
Generic name: zanidatamab-hrii
Dosage form: injection, powder, lyophilized, for solution
Drug class: HER2 inhibitors
Medically reviewed by Drugs.com. Last updated on Nov 28, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ZIIHERA® (zanidatamab‑hrii) for injection, for intravenous use.
Initial U.S. Approval: 2024
WARNING: EMBRYO‑FETAL TOXICITY
See full prescribing information for complete boxed warning.
- •
- Exposure to ZIIHERA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception. (5.1)
Indications and Usage for Ziihera
ZIIHERA is a bispecific HER2-directed antibody indicated for the treatment of adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer (BTC), as detected by an FDA-approved test. (1)
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
Ziihera Dosage and Administration
- •
- Premedicate patients with acetaminophen, an antihistamine and a corticosteroid, 30‑60 minutes prior to each administration of ZIIHERA infusion to prevent potential infusion-related reactions (IRRs). (2.2)
- •
- The recommended dosage of ZIIHERA is 20 mg/kg given as an intravenous infusion once every 2 weeks. (2.3)
Dosage Forms and Strengths
For injection: 300 mg lyophilized powder in a single-dose vial. (3)
Contraindications
- •
- None. (4)
Warnings and Precautions
- •
- Left Ventricular Dysfunction: Assess left ventricular ejection fraction (LVEF) prior to initiation of ZIIHERA and at regular intervals during treatment. Withhold or permanently discontinue ZIIHERA based on severity. (2.4, 5.2)
- •
- Infusion-Related Reactions (IRRs): Premedicate before each infusion of ZIIHERA. Interrupt the infusion, decrease the infusion rate, and/or permanently discontinue ZIIHERA based on severity. (2.4, 5.3)
- •
- Diarrhea: ZIIHERA can cause severe diarrhea. Administer antidiarrheal treatment as clinically indicated. Withhold or permanently discontinue ZIIHERA based on severity. (2.4, 5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (≥ 20%) are diarrhea, infusion-related reaction, abdominal pain, and fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Jazz Pharmaceuticals, Inc. at 1‑800‑520‑5568 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- •
- Females and Males of Reproductive Potential: Verify the pregnancy status of females prior to initiation of ZIIHERA. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2024
Full Prescribing Information
WARNING: EMBRYO-FETAL TOXICITY
Embryo-Fetal Toxicity: Exposure to ZIIHERA during pregnancy can cause embryo-fetal harm. Advise patients of the risk and need for effective contraception [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
1. Indications and Usage for Ziihera
ZIIHERA is indicated for the treatment of adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer (BTC), as detected by an FDA-approved test [see Dosage and Administration (2.1)].
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2. Ziihera Dosage and Administration
2.1 Patient Selection
Select patients for treatment of unresectable or metastatic biliary tract cancer based on HER2-positive (IHC 3+) tumor specimens, as detected by an FDA-approved test [see Clinical Studies (14)].
Information on FDA-approved tests for HER2 protein expression in biliary tract cancers is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Premedications
Premedicate all patients 30 to 60 minutes prior to each dose of ZIIHERA to reduce the risk of infusion-related reactions [see Warnings and Precautions (5.3)]:
- •
- Administer acetaminophen, an antihistamine (such as diphenhydramine) and a corticosteroid (such as hydrocortisone).
2.3 Recommended Dosage
Recommended Dosage and Administration
The recommended dosage of ZIIHERA is 20 mg/kg, administered as an intravenous infusion once every 2 weeks until disease progression or unacceptable toxicity.
Missed dose
If a planned dose of ZIIHERA is delayed or missed, administer the dose as soon as possible; do not wait until the next planned dose. Adjust the administration schedule to maintain a 2-week interval between doses.
2.4 Dosage Modifications for Adverse Reactions
- •
- The recommended dosage reduction of ZIIHERA for adverse reactions is 15 mg/kg as described in Table 1.
- •
- Permanently discontinue ZIIHERA in patients who cannot tolerate 15 mg/kg.
| Adverse Reaction | Severity | Treatment Modification |
|---|---|---|
|
Left Ventricular Dysfunction (LVD) [see Warnings and Precautions (5.2)] |
Absolute decrease of ≥ 16% points in LVEF from pre-treatment baseline or LVEF ≤ 50% and absolute decrease of ≥ 10% points below pre-treatment baseline |
|
|
Confirmed symptomatic congestive heart failure |
|
|
|
Infusion-Related Reactions [see Warnings and Precautions (5.3)] |
Mild (Grade 1) |
|
|
Moderate (Grade 2) |
|
|
|
Severe (Grade 3) |
|
|
|
Life threatening (Grade 4) |
|
|
|
Diarrhea [see Warnings and Precautions (5.4)] |
Mild/Moderate (Grade 1 or 2) |
|
|
Severe (Grade 3) |
|
|
|
Life threatening (Grade 4) |
|
|
|
Pneumonitis [see Adverse Reactions (6.1)] |
Confirmed Grade ≥ 2 |
|
|
Other Adverse Reactions (excluding LVD, IRR, Diarrhea, and Pneumonitis) [see Adverse Reactions (6.1)] |
Mild/Moderate (Grades 1/2) |
|
|
Severe (Grade 3) |
|
|
|
Life Threatening (Grade 4) |
|
2.5 Preparation and Administration Instructions
Administer only as an intravenous infusion after ZIIHERA is reconstituted and diluted.
Reconstitution
- •
- Calculate the recommended dose based on the patient’s weight to determine the number of vials needed.
- •
- Remove the vial(s) from the refrigerator and allow the vial(s) to reach room temperature.
- •
- Reconstitute each 300 mg vial of ZIIHERA with 5.7 mL of Sterile Water for Injection by slowly directing the stream toward the inside of the wall of the vial, to obtain a final concentration of 50 mg/mL in an extractable volume of 6 mL.
- •
- Swirl the vial gently until completely dissolved. Do not shake or vigorously swirl.
- •
- Allow the reconstituted vial to settle to allow bubbles to dissipate.
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted product should be a colorless to light yellow, clear to slightly opalescent solution with no visible particles. Discard the reconstituted vial if any discoloration or particulate matter is observed.
- •
- The product does not contain a preservative. Use the reconstituted ZIIHERA solution immediately or store the reconstituted ZIIHERA solution for up to 4 hours, either at room temperature (18°C to 24°C [64°F to 75°F]) or in a refrigerator (2°C to 8ºC [36°F to 46ºF]).
Dilution
- •
- Withdraw the necessary volume for the calculated dose from each vial [see Dosage and Administration (2.3)].
- •
- Slowly add the necessary dose volume to an infusion bag containing 0.9% Sodium Chloride Injection or 5% Dextrose Injection to prepare an infusion solution with a final concentration of the diluted solution between 0.4 mg/mL and 6 mg/mL.
- •
- Gently invert the infusion bag to mix. Do not shake.
- •
- The infusion solution must be a clear, colorless solution with no visible particles. Do not use if visible particles are observed or if the solution is discolored.
- •
- Discard any unused portion left in the vial(s).
- •
- Use the infusion solution immediately upon dilution or store the infusion solution at room temperature (18°C to 24°C [64°F to 75°F]) for up to 12 hours or in the refrigerator (2ºC to 8ºC [36ºF to 46ºF]) for up to 24 hours.
- o
- These time limits include the beginning of reconstitution through the duration of infusion.
- o
- If these specified times are exceeded, discontinue the current infusion bag and prepare a new bag which contains the remaining dosage of ZIIHERA to be infused.
- •
- Compatibility with intravenous administration materials and the infusion solution has been demonstrated in the following materials:
- o
- Intravenous (IV) Bag: Polyvinyl chloride (PVC), polyolefin (PO), ethyl vinyl acetate (EVA), polypropylene (PP) and ethylene-propylene copolymer.
- o
- Infusion sets: Polyvinyl chloride/ bis (2-ethylhexyl) phthalate (PVC/DEHP). Polyurethane (PUR), polyethylene-lined (PE-lined) acrylonitrile-butadiene-styrene (ABS).
- o
- Inline filters: Polyethersulfone solution filter (PES), polyvinylidene fluoride air filter (PVDF).
- o
- Closed System Transfer devices: acrylonitrile-butadiene-styrene (ABS), acrylic c-polymer, polycarbonate (PC), polyisoprene (PI), polyester, polypropylene (PP) polytetrafluoroethylene (PTFE), silicone and stainless steel (SS).
Administration
- •
- Administer ZIIHERA as an intravenous infusion with a 0.2 or 0.22 micron filter.
- •
- Do not administer as an intravenous push or bolus.
- •
- Do not co-administer ZIIHERA and other intravenous drugs through the same intravenous line.
|
Dose |
Infusion Duration |
|
First and Second |
120-150 minutes |
|
Third and Fourth |
90 minutes, if previous infusions were well-tolerated |
|
Subsequent |
60 minutes, if previous infusions were well-tolerated |
5. Warnings and Precautions
5.1 Embryo-Fetal Toxicity
Based on the mechanism of action, ZIIHERA can cause fetal harm when administered to a pregnant woman. There are no human or animal data on the use of ZIIHERA in pregnancy. In literature reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
Verify the pregnancy status of females of reproductive potential prior to the initiation of ZIIHERA. Advise pregnant women and females of reproductive potential that exposure to ZIIHERA during pregnancy or within 4 months prior to conception can result in fetal harm. Advise females of reproductive potential to use effective contraception while receiving ZIIHERA and for 4 months following the last dose of ZIIHERA.
5.2 Left Ventricular Dysfunction
ZIIHERA can cause decreases in left ventricular ejection fraction (LVEF). LVEF declined by greater than 10% and decreased to less than 50% in 4.3% of 233 patients. LVD leading to permanent discontinuation of ZIIHERA was reported in 0.9% of patients. The median time to first occurrence of left ventricular dysfunction was 5.6 months (range: 1.6 to 18.7 months). Left ventricular dysfunction resolved in 70% of patients.
Assess LVEF prior to initiation of ZIIHERA and at regular intervals during treatment. Withhold dose or permanently discontinue ZIIHERA based on severity of adverse reactions [see Dosage and Administration (2.4)].
The safety of ZIIHERA has not been established in patients with a baseline ejection fraction that is below 50% [see Dosage and Administration (2.4)].
5.3 Infusion-Related Reactions
ZIIHERA can cause infusion-related reactions (IRRs). An IRR was reported in 31% of 233 patients treated with ZIIHERA as a single agent in clinical studies, including Grade 3 (0.4%), and Grade 2 (25%). IRRs leading to permanent discontinuation of ZIIHERA were reported in 0.4% of patients. IRRs occurred on the first day of dosing in 28% of patients; 97% of IRRs resolved within one day.
Prior to each dose of ZIIHERA, administer premedications to prevent potential IRRs [see Dosage and Administration (2.2)]. Monitor patients for signs and symptoms of IRR during ZIIHERA administration and as clinically indicated after completion of infusion. Have medications and emergency equipment to treat IRRs available for immediate use.
If an IRR occurs, slow, or stop the infusion, and administer appropriate medical management. Monitor patients until complete resolution of signs and symptoms before resuming. Permanently discontinue ZIIHERA in patients with recurrent severe or life-threatening infusion-related reactions [see Dosage and Administration (2.4)].
5.4 Diarrhea
ZIIHERA can cause severe diarrhea [see Adverse Reactions (6.1)].
Diarrhea was reported in 48% of 233 patients treated in clinical studies, including Grade 3 (6%) and Grade 2 (17%). If diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Withhold or permanently discontinue ZIIHERA based on severity [see Dosage and Administration (2.4)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described in greater detail in other sections of the labeling:
- •
- Embyro-Fetal Toxicity [see Warnings and Precautions (5.1)]
- •
- Left Ventricular dysfunction [see Warnings and Precautions (5.2)]
- •
- Infusion-Related Reactions [see Warnings and Precautions (5.3)]
- •
- Diarrhea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population of ZIIHERA described in WARNINGS AND PRECAUTIONS reflect exposure in 233 patients administered ZIIHERA 20 mg/kg intravenously as a single agent in two single-arm, open-label studies (ZWI‑ZW25‑101 and HERIZON‑BTC‑01), which enrolled 109 patients with biliary tract cancer, and 124 patients with other cancers. Among 233 patients who received ZIIHERA, 39% were exposed for 6 months or longer, and 17% were exposed for greater than one year.
Biliary Tract Cancer
The safety of ZIIHERA was evaluated in 80 patients with previously treated, unresectable or metastatic HER2-positive biliary tract cancer who received at least one prior gemcitabine-containing chemotherapy regimen in HERIZON‑BTC‑01 [See Clinical Studies (14)]. Patients received ZIIHERA 20 mg/kg by IV infusion once every 2 weeks until disease progression or unacceptable toxicity. The median duration of exposure to ZIIHERA was 5.6 months (range: 0.5 to 27.2 months).
Serious adverse reactions occurred in 53% of patients who received ZIIHERA. Serious adverse reactions in > 2% of patients included biliary obstruction (15%), biliary tract infection (8%), sepsis (8%), pneumonia (5%), diarrhea (3.8%), gastric obstruction (3.8%), and fatigue (2.5%). A fatal adverse reaction of hepatic failure occurred in one patient who received ZIIHERA.
Permanent discontinuation due to an adverse reaction occurred in 2.5% of patients who received ZIIHERA. Adverse reactions which resulted in permanent discontinuation in ≥ 1% of patients who received ZIIHERA included decreased ejection fraction and pneumonitis.
Dosage interruptions due to an adverse reaction, excluding temporary interruptions of ZIIHERA infusions due to infusion-related reactions, occurred in 41% of patients who received ZIIHERA. The most frequent adverse reactions (> 2% of patients) that required dosage interruption were diarrhea, increased alanine aminotransferase, increased aspartate aminotransferase, decreased ejection fraction, pneumonia, cholangitis, fatigue, biliary obstruction, abdominal pain, increased blood creatinine, and decreased potassium.
Dosage reductions due to an adverse reaction occurred in 4% of patients who received ZIIHERA. Adverse reactions requiring dosage reductions in > 1% of patients were diarrhea, nausea, and decreased weight.
The most common adverse reactions in patients receiving ZIIHERA (≥ 20%) were diarrhea, infusion-related reaction, abdominal pain, and fatigue.
Table 3 summarizes the adverse reactions that occurred in HERIZON‑BTC‑01.
|
Adverse Reaction* |
ZIIHERA |
|
|
All Grades |
Grades 3 or 4 |
|
|
Gastrointestinal disorders |
||
|
Diarrheaa |
50 |
10 |
|
Abdominal painb |
29 |
1 |
|
Nausea |
18 |
1 |
|
Vomiting |
15 |
1 |
|
Injury, poisoning and procedural complications |
||
|
Infusion-related reaction |
35 |
1 |
|
General disorders and administration site conditions |
||
|
Fatiguec |
24 |
4 |
|
Skin and subcutaneous tissue disorders |
||
|
Rashd |
19 |
0 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
16 |
0 |
* Graded per CTCAE version 5.
a Diarrhea includes diarrhea and enteritis
b Abdominal pain includes abdominal pain and abdominal pain upper
c Fatigue includes asthenia and fatigue
d Rash includes dermatitis, dermatitis acneiform, palmar-plantar erythrodysaesthesia syndrome, rash, rash maculo-papular, and rash pustular
Table 4 summarizes the laboratory abnormalities in HERIZON‑BTC‑01.
|
Laboratory Abnormalities |
ZIIHERA* |
|
|
All Grades |
Grades 3 or 4 |
|
|
Hematology | ||
|
88 |
14 |
|
44 |
8 |
|
Chemistry | ||
|
55 |
0 |
|
53 |
0 |
|
47 |
10 |
|
46 |
8 |
|
41 |
5 |
|
35 |
10 |
|
34 |
5 |
*The denominator used to calculate the rate varied from 78 to 80 based on the number of patients with a baseline value and at least one post-treatment value.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on mechanism of action, ZIIHERA can cause fetal harm when administered to a pregnant woman. There are no human or animal data on the use of ZIIHERA in pregnancy. In literature reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death. Use of ZIIHERA is not recommended during pregnancy (see CLINICAL CONSIDERATIONS). Advise patients of potential risks to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Monitor women who received ZIIHERA during pregnancy or within 4 months prior to conception for oligohydramnios. If oligohydramnios occurs, perform fetal testing that is appropriate for gestational age and consistent with local standard of care.
8.2 Lactation
Risk Summary
There are no data on the presence of zanidatamab‑hrii in human milk, the effects on the breastfed child, or the effects on milk production. Published data suggest human IgG is present in human milk but does not enter neonatal or infant circulation in substantial amounts. Consider developmental and health benefits of breastfeeding along with the mother’s clinical need for ZIIHERA treatment and any potential adverse effects on the breastfed child from ZIIHERA or from the underlying maternal condition. This consideration should also take into account the ZIIHERA half-life of approximately 21 days and a washout period of 4 months [see Clinical Pharmacology (12.3)].
8.3 Females and Males of Reproductive Potential
ZIIHERA can cause fetal harm when administered to a pregnant woman [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of ZIIHERA [see Use in Specific Populations (8.1)].
Contraception
Females
ZIIHERA can cause embryo-fetal harm when administered during pregnancy. Advise females of reproductive potential to use effective contraception during treatment with ZIIHERA and for 4 months following the last dose of ZIIHERA.
8.5 Geriatric Use
Of the 80 patients who received ZIIHERA for unresectable or metastatic biliary tract cancer in HERIZON‑BTC‑01, there were 39 (49%) patients 65 years of age and older. Thirty‑seven (46%) were aged 65‑74 years old and 2 (3%) were aged 75 years or older [see Clinical Studies (14)].
No overall differences in safety or effectiveness were observed between these patients and younger adult patients.
11. Ziihera Description
Zanidatamab‑hrii is a humanized, IgG-like, bispecific HER2-directed antibody. Zanidatamab‑hrii is produced in Chinese hamster ovary cells via recombinant DNA technology and has a molecular weight of 124.8 kDa.
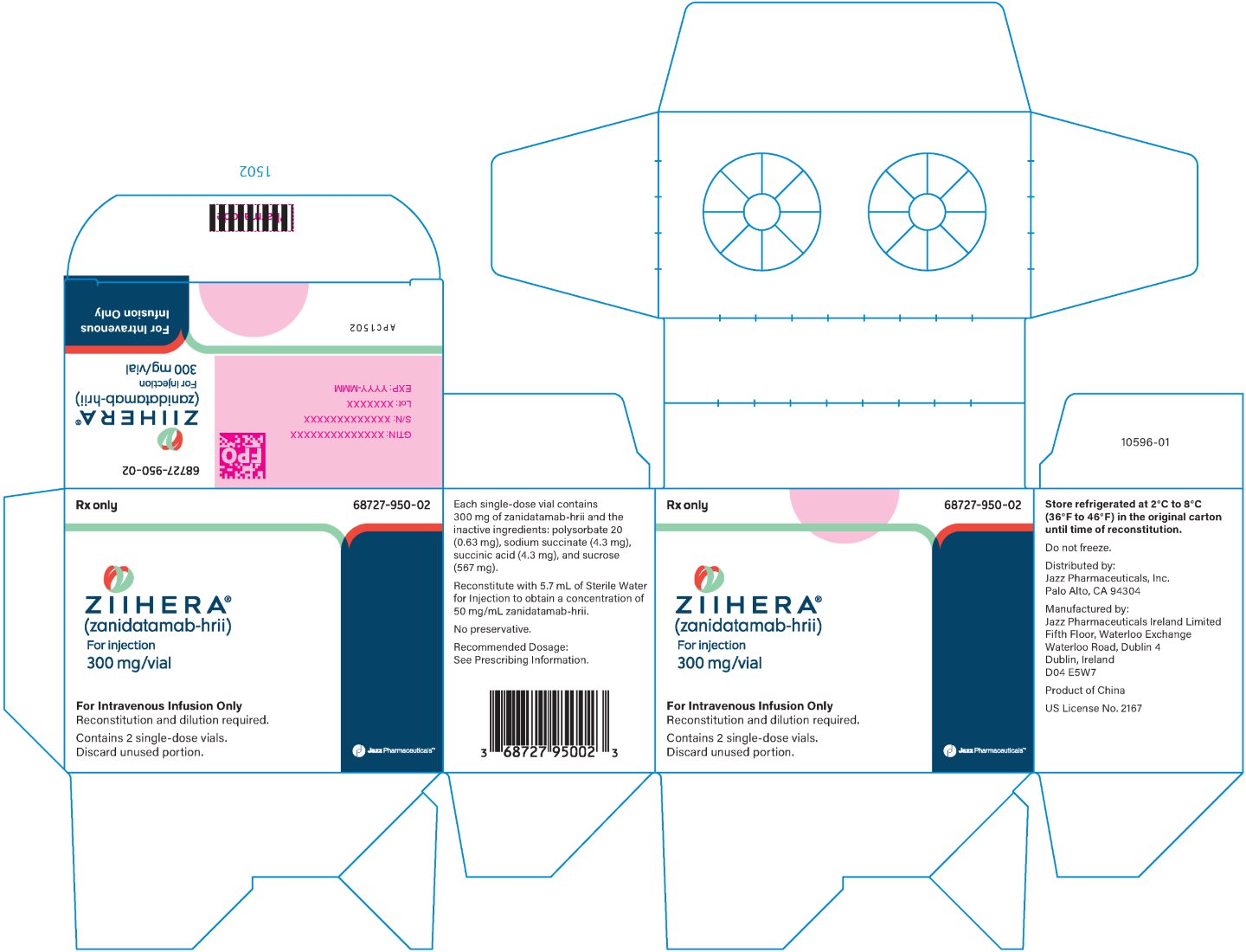
ZIIHERA (zanidatamab‑hrii) for injection is supplied as a sterile, preservative free, white lyophilized powder that requires reconstitution and dilution for intravenous use. Each single-dose vial of reconstituted product contains 300 mg of zanidatamab‑hrii and the inactive ingredients: polysorbate 20 (0.63 mg), sodium succinate (4.3 mg), succinic acid (4.3 mg), and sucrose (567 mg). Following reconstitution with 5.7 mL Sterile Water for Injection, a solution containing 50 mg/mL zanidatamab‑hrii is produced with a deliverable volume of 6 mL, with pH of 4.6. The resulting solution is diluted and administered by intravenous infusion.
12. Ziihera - Clinical Pharmacology
12.1 Mechanism of Action
Zanidatamab‑hrii is a bispecific HER2-directed antibody that binds to two extracellular sites on HER2. Binding of zanidatamab‑hrii with HER2 results in internalization leading to a reduction of the receptor on the tumor cell surface. Zanidatamab‑hrii induces complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). These mechanisms result in tumor growth inhibition and cell death in vitro and in vivo.
12.2 Pharmacodynamics
Zanidatamab‑hrii exposure-response relationships and the time course of the pharmacodynamic response are unknown.
Cardiac Electrophysiology
A mean increase in the QTc interval > 20 ms was not observed at the recommended approved dosage.
12.3 Pharmacokinetics
Zanidatamab‑hrii PK parameters are presented as means (percent coefficient of variation) following administration of ZIIHERA 20 mg/kg every 2 weeks after the 7th or later dose unless otherwise indicated.
Zanidatamab‑hrii maximum concentration (Cmax) is 600 (22.2) µg/mL, the lowest measured concentration (Ctrough) is 178 (29.6) µg/mL, and total systemic exposure (AUC0-336h) is 3,976 (22.5) days*µg/mL following administration of ZIIHERA. The Cmax of zanidatamab‑hrii is dose proportional and the total systemic exposure (AUC0-inf) of zanidatamab‑hrii is greater than dose proportional with increasing doses. The mean Ctrough accumulation ratio of zanidatamab‑hrii is approximately 2.4.
Distribution
The volume of distribution of zanidatamab‑hrii is approximately 7.5 (33) L based on the population pharmacokinetic analysis.
Elimination
The estimated half-life (t1/2) of zanidatamab‑hrii is approximately 21 days with an associated clearance (CL) of 0.012 L/h (27.9) based on the population pharmacokinetic analysis.
Metabolism
Zanidatamab‑hrii is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of zanidatamab-hrii were observed based on age (24 to 88 years), sex, race (White and Asian), mild and moderate renal impairment (eGFR 30 to 89 mL/min estimated using the CKD‑EPI), mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and AST > ULN or total bilirubin between 1 and 1.5 times ULN and any AST), or body weight (35 kg to 128 kg).
The effect of severe renal impairment (eGFR 15 to 29 mL/min), end-stage renal disease (eGFR < 15 mL/min) with or without hemodialysis, and moderate (total bilirubin > 1.5 to ≤ 3 ULN and any AST) or severe (total bilirubin > 3 ULN and any AST) hepatic impairment on the pharmacokinetics of zanidatamab‑hrii is unknown.
14. Clinical Studies
HER2-positive (IHC 3+) Biliary Tract Cancer (BTC)
The efficacy of ZIIHERA was evaluated in 62 patients with HER2-positive (IHC 3+ by central assessment) BTC in Cohort 1 of HERIZON‑BTC‑01 (NCT04466891), an open-label, multicenter, single arm trial in patients with unresectable or metastatic disease. Patients were required to have received at least one prior gemcitabine-containing systemic chemotherapy regimen in the advanced disease setting and adequate cardiac function (defined as LVEF ≥ 50%).
Patients received ZIIHERA 20 mg/kg intravenously every 2 weeks. ZIIHERA was administered until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) and duration of response (DOR) as determined by an independent central review (ICR) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1.
The median age was 64 years (range: 38 to 79 years), 47% of patients were age 65 or older; 55% were female; 61% were Asian, 31% were White, 2% were American Indian or Alaskan Native and for 6% race was unknown or not reported; 89% were Non-Hispanic or Latino, 8% Hispanic/Latino, and for 3% ethnicity was unknown or not reported. All patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 (32%) or 1 (68%). Fifty-three percent of patients had gallbladder cancer, 27% had intrahepatic cholangiocarcinoma, and 19% had extrahepatic cholangiocarcinoma. All patients received at least 1 prior line of gemcitabine-based therapy, 31% had 2 prior lines of therapy, and 10% had 3 or more prior lines of therapy for unresectable or metastatic disease.
Efficacy results are summarized in Table 5.
|
Efficacy Parameter* |
ZIIHERA |
|
Objective Response Rate (95% CI) |
52% (39, 65) |
|
2 (3.2) |
|
30 (48) |
|
Duration of Response (DOR) |
N=32 |
|
14.9 (7.4, NE) |
|
(19 59) |
|
14 (44) |
*Assessed by independent central review
† Based on Kaplan-Meier estimate
‡ Based on observed duration of response
NE = not estimable
16. How is Ziihera supplied
ZIIHERA is supplied as a sterile, preservative free, white lyophilized powder in a single-dose vial. Each single-dose vial (NDC 68727‑950‑01) contains 300 mg zanidatamab‑hrii. Each carton of ZIIHERA (NDC 68727‑950‑02) contains 2 single-dose vials.
Store in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF) in the original carton until time of reconstitution. Do not freeze.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryo-Fetal Toxicity
Advise female patients of the potential risk to a fetus. Advise female patients to contact their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with ZIIHERA and for 4 months following the last dose [see Warnings and Precautions (5.1), Use in Specific Populations (8.3)].
Left Ventricular Dysfunction
Advise patients that ZIIHERA can cause cardiac dysfunction and to contact a healthcare provider immediately for signs and symptoms of cardiac dysfunction [see Warnings and Precautions (5.2)].
Infusion-Related Reactions
Advise patients of the risk of infusion-related reactions and to inform a healthcare provider immediately for symptoms of an infusion-related reaction [see Warnings and Precautions (5.3)].
Diarrhea
Advise patients that ZIIHERA can cause diarrhea, which may be severe. Instruct patients how to manage diarrhea, and to contact their healthcare provider for sustained diarrhea that does not respond to supportive care [see Dosage and Administration (2.4), Warnings and Precautions (5.4)].
Distributed by:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94304
Manufactured by:
Jazz Pharmaceuticals Ireland Limited
Fifth Floor, Waterloo Exchange
Waterloo Road, Dublin 4
Dublin, Ireland
D04 E5W7
U.S. License No. 2167
|
PATIENT INFORMATION |
|
What is the most important information I should know about ZIIHERA? ZIIHERA can cause serious side effects, including:
See “What are the possible side effects of ZIIHERA?” for more information about side effects. |
|
What is ZIIHERA? ZIIHERA is a prescription medicine used to treat adults who have a type of bile duct (cholangiocarcinoma) or gallbladder cancer called biliary tract cancer (BTC), that is human epidermal growth factor receptor 2 (HER2)-positive (IHC 3+). ZIIHERA may be used when your BTC:
Your healthcare provider will perform a test to make sure ZIIHERA is right for you. It is not known if ZIIHERA is safe and effective in children. |
|
Before receiving ZIIHERA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
|
How should I receive ZIIHERA?
|
|
What are the possible side effects of ZIIHERA? ZIIHERA can cause serious side effects, including:
Other common side effects of ZIIHERA include stomach pain and feeling tired. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of ZIIHERA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088. |
|
General information about the safe and effective use of ZIIHERA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about ZIIHERA that is written for health professionals. |
|
What are the ingredients in ZIIHERA?
Active ingredient: zanidatamab-hrii Distributed by: Manufactured by: U.S. License No. 2167 For more information, go to www.ZIIHERA.com or call 1‑800‑520‑5568. |
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Issued: 11/2024 |
| ZIIHERA
zanidatamab-hrii injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Jazz Pharmaceuticals, Inc. (135926363) |
| Registrant - Jazz Pharmaceuticals Ireland Limited (896650210) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| WuXi Biologics Co., Ltd. | 421298354 | MANUFACTURE(68727-950) , ANALYSIS(68727-950) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Almac Pharma Services LLC | 078607239 | PACK(68727-950) , LABEL(68727-950) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jazz Pharmaceuticals Ireland Limited | 896650210 | MANUFACTURE(68727-950) | |
Biological Products Related to Ziihera
Find detailed information on biosimilars for this medication.
More about Ziihera (zanidatamab)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: HER2 inhibitors
- En español