Zaditor: Package Insert / Prescribing Info
Package insert / product label
Generic name: ketotifen fumarate
Dosage form: Ophthalmic Solution, 0.025%
Drug class: Ophthalmic antihistamines and decongestants
Medically reviewed by Drugs.com. Last updated on Mar 24, 2025.
On This Page
Zaditor Description
ZADITOR™ is a sterile ophthalmic solution containing ketotifen for topical administration to the eyes. Ketotifen fumarate is a finely crystalline powder with an empirical formula of C23H23NO5S and a molecular weight of 425.50.
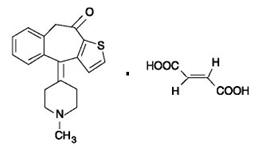
Established Name: ketotifen fumarate ophthalmic solution
CHEMICAL NAME
4-(1-Methyl-4-piperidylidene)-4H-benzo[4,5]cyclohepta[1,2-b] thiophen-10(9H)-one hydrogen fumarate
Each mL of ZADITOR™ contains: Active: 0.345 mg ketotifen fumarate equivalent to 0.25 mg ketotifen. Inactives: glycerol, sodium hydroxide/hydrochloric acid (to adjust pH) and purified water.
Preservative: benzalkonium chloride 0.01%. It has a pH of 4.4 to 5.8 and an osmolality of 210-300 mOsm/kg.
Zaditor - Clinical Pharmacology
Ketotifen is a relatively selective, non-competitive histamine antagonist (H1-receptor) and mast cell stabilizer. Ketotifen inhibits the release of mediators from cells involved in hypersensitivity reactions. Decreased chemotaxis and activation of eosinophils has also been demonstrated.
Ketotifen has been shown to have little systemic exposure following topical ocular administration. A study conducted with 15 healthy volunteers dosed bilaterally with ketotifen fumarate ophthalmic solution twice daily for 14 days demonstrated plasma concentrations generally below the quantitation limit of assay (< 20 pg/mL).
In human conjunctival allergen challenge studies, ZADITOR™ was significantly more effective than placebo in preventing ocular itching associated with allergic conjunctivitis. The action of ketotifen occurs rapidly with an effect seen within minutes after administration.
Indications and Usage for Zaditor
ZADITOR™ (ketotifen fumarate ophthalmic solution) is indicated for the temporary prevention of itching of the eye due to allergic conjunctivitis.
Contraindications
ZADITOR™ is contraindicated in persons with a known hypersensitivity to any component of this product.
Precautions
Information for patients
To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep the bottle tightly closed when not in use. Patients should be advised not to wear a contact lens if their eye is red. ZADITOR™ should not be used to treat contact lens related irritation. The preservative in ZADITOR™, benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red, should be instructed to wait at least ten minutes after instilling ZADITOR™ before they insert their contact lenses.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Ketotifen fumarate was determined to be non-mutagenic in a battery of in vitro and in vivo mutagenicity assays including: Ames test, in vitro chromosomal aberration test with V79 Chinese hamster cells, in vivo micronucleus assay in mouse, and mouse dominant lethal test.
Treatment of male rats with oral doses of ketotifen ≥ 10 mg/kg/day orally [6,667 times the maximum recommended human ocular dose of 0.0015 mg/kg/day on a mg/kg basis (MRHOD)] for 70 days prior to mating resulted in mortality and a decrease in fertility. Treatment with ketotifen did not impair fertility in female rats receiving up to 50 mg/kg/day of ketotifen orally (33,333 times the MRHOD) for 15 days prior to mating.
Pregnancy
Pregnancy Category C
Oral treatment of pregnant rabbits during organogenesis with 45 mg/kg/day of ketotifen (30,000 times the MRHOD) resulted in an increased incidence of retarded ossification of the sternebrae. However, no effects were observed in rabbits treated with up to 15 mg/kg/day (10,000 times the MRHOD). Similar treatment of rats during organogenesis with 100 mg/kg/day of ketotifen (66,667 times the MRHOD) did not reveal any biologically relevant effects.
Oral treatment of pregnant rats (up to 100 mg/kg/day or 66,667 times the MRHOD) and rabbits (up to 45 mg/kg/day or 30,000 times the MRHOD) during organogenesis did not result in any biologically relevant embryofetal toxicity. In the offspring of the rats that received ketotifen orally from day 15 of pregnancy to day 21 post partum at 50 mg/kg/day (33,333 times the MRHOD), a maternally toxic treatment protocol, the incidence of postnatal mortality was slightly increased, and body weight gain during the first four days post partum was slightly decreased.
Nursing Mothers
Ketotifen fumarate has been identified in breast milk in rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, caution should be exercised when ketotifen fumarate is administered to a nursing mother.
Adverse Reactions/Side Effects
In controlled clinical studies, conjunctival injection, headaches, and rhinitis were reported at an incidence of 10 to 25%. The occurrence of these side effects was generally mild. Some of these events were similar to the underlying ocular disease being studied.
The following ocular and non-ocular adverse reactions were reported at an incidence of less than 5%:
Ocular: Allergic reactions, burning or stinging, conjunctivitis, discharge, dry eyes, eye pain, eyelid disorder, itching, keratitis, lacrimation disorder, mydriasis, photophobia, and rash.
Non-Ocular: Flu syndrome, pharyngitis.
Overdosage
Oral ingestion of the contents of a 5 mL bottle would be equivalent to 1.725 mg of ketotifen fumarate. Clinical results have shown no serious signs or symptoms after the ingestion of up to 20 mg of ketotifen fumarate.
Zaditor Dosage and Administration
The recommended dose is one drop in the affected eye(s) twice daily, every 8 to 12 hours.
| ZADITOR
ketotifen fumarate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Novartis Ophthalmics |
More about Zaditor (ketotifen ophthalmic)
- Compare alternatives
- Pricing & coupons
- Reviews (9)
- Side effects
- Dosage information
- During pregnancy
- Drug class: ophthalmic antihistamines and decongestants
- Breastfeeding
- En español
