Ultrasal-ER: Package Insert / Prescribing Info
Package insert / product label
Generic name: salicylic acid
Dosage form: topical solution
Medically reviewed by Drugs.com. Last updated on Dec 2, 2024.
On This Page
Ultrasal-ER (28.5% Salicylic Acid Extended Release) Antiviral Film-Forming Solution: Package Insert
DESCRIPTION
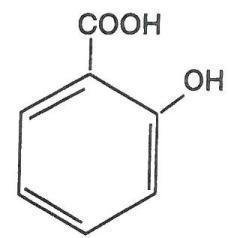
UltraSal-ER is a topical preparation containing 28.5% salicylic acid extended release in a proprietary film-forming virucidal solution composed of acrylates copolymer, butyl acetate, carthamus tinctorius seed oil, cocamidopropyl dimethylamine, ethylhexylglycerin, isopropyl alcohol, isopropyl-metacresol, olea europaea fruit oil, phenic acid, phenoxyethanol, polysorbate 80, polyvinyl butyral, trimethyl pentanyl diisobutyrate, and water. The pharmacologic activity of UltraSal-ER is generally attributed to the keratolytic activity of salicylic acid, which is incorporated into a polyacrylic, film-forming virucidal solution designed to cover the wart without the need for a bandage. The structural formula of salicylic acid is:
CLINICAL PHARMACOLOGY
Although the exact mode of action for salicylic acid in the treatment of warts is unknown, its activity appears to be associated with its keratolytic action, which results in mechanical removal of epidermal cells infected with wart viruses. UltraSal-ER incorporates a unique patented extended release form of salicylic acid that provides for enhanced release of salicylic acid for over 24 hours.
The virucidal complex incorporated into UltraSal-ER’s proprietary solution is designed to help reduce risk of reinfection at the wart site, as well as prevent viral contamination of the product under normal usage.
INDICATIONS AND USAGE
UltraSal-ER is indicated for the topical treatment and removal of common warts and plantar warts.
CONTRAINDICATIONS
Patients with diabetes or impaired blood circulation should not use UltraSal-ER. UltraSal-ER also should not be used on moles, birthmarks, and unusual warts with hair growing from them, or warts on the face.
PRECAUTIONS
UltraSal-ER is for external use only. Do not permit UltraSal-ER to contact eyes or mucous membranes. If contact with eyes or mucous membranes occurs, immediately flush with water for 15 minutes. UltraSal-ER should not be allowed to contact normal skin surrounding the wart site, since localized irritation may occur. Treatment should be discontinued if excessive irritation occurs.
UltraSal-ER is flammable. Keep away from fire or flame. Keep bottle tightly capped when not in use.
ADVERSE REACTIONS
A localized irritant reaction may occur if Ultrasal-ER is applied to the normal skin surrounding the wart. Any irritation may normally be controlled by temporarily discontinuing use and by applying the medication only to the wart site when treatment is resumed.
DOSAGE AND ADMINISTRATION
Prior to applying UltraSal-ER, soak wart in warm water for five minutes. Remove any loose tissue by gently rubbing with a washcloth, emery board, or pumice stone. Dry the wart site thoroughly. Using the brush applicator supplied, apply UltraSal-ER twice to the entire wart surface, allowing the first application to dry before applying the second. Continue treatment once or twice a day as directed by your healthcare provider. Be careful not to apply to surrounding skin.
Clinically visible improvement normally occurs during the first or second week of therapy. Resolution may be expected after four to six weeks of UltraSal-ER use, though some warts may take longer to remove.
HOW SUPPLIED
UltraSal-ER is supplied in 10 mL amber bottles with a brush applicator (NDC 42783-323-10).
Store at 15° to 30°C (59° to 86°F).
Manufactured for:
Elorac, Inc.
Vernon Hills, IL 60061
U.S. Patent No. 6,979,440
Additional Patent Pending
© 2014 Elorac, Inc.
1/2014
211909
PATIENT INSTRUCTIONS
Your health care provider has prescribed UltraSal-ER, a topical prescription preparation for the treatment of common warts and plantar warts. In order for UltraSal-ER to work properly and to ensure maximum benefit, the following instructions should be followed carefully. Of course, as with any medication, always consult your health care provider if you experience any discomfort or unexpected reactions.
STEP 1
WASH
- Soak wart area in warm water for about 5 minutes.
- Remove any loose tissue using a washcloth, emery board, or pumice stone.
- Dry thoroughly.
STEP 2
BRUSH
- Using the brush applicator supplied, apply twice to entire wart surface, allowing the first application to dry before applying the second.
- Be careful not to apply to surrounding skin.
- Leave UltraSal-ER on wart area after it dries.
Repeat Step 1 and Step 2 once or twice daily as advised by the health care provider.
Clinically visible improvement will normally occur during the first or second week of therapy. Resolution may be expected after four to six weeks of UltraSal-ER use, though some warts may take longer to remove.

Vernon Hills, IL 60061
| ULTRASAL-ER
salicylic acid solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Elorac, Inc. (832590009) |
| Registrant - Elorac, Inc. (832590009) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Swiss-American CDMO, LLC | 080170933 | manufacture(42783-323) , analysis(42783-323) , pack(42783-323) , label(42783-323) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Microconsult, Inc. | 062183608 | analysis(42783-323) | |
Frequently asked questions
More about UltraSal-ER (salicylic acid topical)
Patient resources
Professional resources
Other brands
Salex, Salvax, Keralyt Shampoo, KeralytGel, ... +5 more


