Sodium Chloride Intravenous Infusion: Package Insert / Prescribing Info
Package insert / product label
Dosage form: intravenous infusion
Drug classes: Minerals and electrolytes, Miscellaneous respiratory agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
IMPORTANT DRUG INFORMATION
March 2014
Subject: Temporary Importation of 0.9% Sodium Chloride Injection in VIAFLO (non-PVC) Containers
Dear Healthcare Professional,
Due to current critical shortage of 0.9% Sodium Chloride Injection in the U.S. market, Baxter Healthcare Corporation (Baxter) is coordinating with the Food and Drug Administration (FDA) to increase the availability of the drug. Baxter has initiated temporary importation of a foreign Sodium Chloride 0.9% Injection Solution for Intravenous Infusion in VIAFLO non-polyvinyl chloride (non-PVC) containers manufactured at Baxter’s Bieffe Medital, Sabinanigo, Spain facility and marketed within the European Union.
At this time, FDA is not objecting to the importation and distribution of Baxter’s Sodium Chloride 0.9% Injection Solution for Intravenous Infusion to address the critical shortage of Sodium Chloride 0.9% Injection. Importation or distribution of Baxter’s Sodium Chloride 0.9% Injection Solution for Intravenous Infusion by any entity other than Baxter is not within the scope of this decision and may be subject to enforcement action by the FDA. FDA has not approved this product in the United States.
Effective immediately, Baxter will offer the Sodium Chloride 0.9% Intravenous Infusion in VIAFLO (non-PVC) containers in the following volumes and quantities:
| Product | VIAFLO (non-PVC) Container |
| Sodium Chloride 0.9% Intravenous Infusion, 250 mL | 30 bags per carton |
| Sodium Chloride 0.9% Intravenous Infusion, 500 mL | 20 bags per carton |
| Sodium Chloride 0.9% Intravenous Infusion, 1000 mL (1L) | 10 bags per carton |
Indications and Usage and Dosage Administration
Baxter’s Sodium Chloride 0.9% Intravenous Infusion in the VIAFLO (non-PVC) containers is the same formulation and concentration of active ingredient (sodium chloride) as the 0.9% Sodium Chloride Injection products currently approved by the U.S. FDA in the VIAFLEX (PVC) and AVIVA (non-PVC) containers. As such, clinical practice pertaining to indication, usage and dosage administration for Sodium Chloride 0.9% Intravenous Infusion in VIAFLO (non-PVC) containers is the same as with VIAFLEX (PVC) and AVIVA (non-PVC) containers.
However, before prescribing, healthcare providers should be aware of some key differences between the VIAFLEX (PVC), AVIVA (non-PVC) and VIAFLO (non-PVC) container packaging and labeling. Healthcare providers should refer to the product package inserts before use. Key differences are highlighted in the attached Product Comparison Tables as follows:
- Table 1: Key Differences in 0.9% Sodium Chloride Products
- Table 2: Comparison of Container Labels
- Table 3: Comparison of Carton Labels
It is also important to note the following:
- The injection/medication ports are similar across the VIAFLEX (PVC), AVIVA (non-PVC), and VIAFLO (non-PVC) containers. The VIAFLO (non-PVC) administration port system is fully compatible with IV set spike heads that meet the International Organization of Standardization (ISO) standards and with Baxter IV sets marketed in the United States.
- Prior to use, it is important to check for leaks by squeezing the inner bag firmly. If leaks are found, discard solution as sterility may be impaired. Additionally, check to see that solution is clear and free of foreign matter. Discard the solution if solution is not clear.
- VIAFLO (non-PVC) imported container and carton labeling includes barcodes; however, the barcodes may not register accurately in the U.S. scanning systems. Institutions should manually input the product into their systems and confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
- Please review container labels carefully to avoid potential volume selection errors. For example, the US-approved Viaflex 1 L product and the imported 500 mL Viaflo product are of similar bag size and shape.
If you have any questions about the information contained in this letter or the use of 0.9% Sodium Chloride Injection in the VIAFLO (non-PVC) container, please contact Baxter’s Medical Information Service at 1-800-933-0303. To place an order, please contact Baxter's Center for Service by calling 1-888-229-0001.
To report product quality issues please contact Baxter Product Surveillance at 1-800-437-5176. To report adverse events associated with 0.9% Sodium Chloride Injection, please call Baxter at 1-866-888-2472, or fax: 1-800-759-1801. Adverse events that may be related to the use of this product may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Online: www.fda.gov/medwatch/report.htm1
-
Regular Mail: use postage-paid FDA form 3500 available at:
www.fda.gov/MedWatch/getforms.htm2. Mail to MedWatch 5600 Fishers Lane, Rockville, MD 20852-9787 - Fax: 1-800-FDA-0178
Sincerely,
Thomas J. Progar
Marketing Strategy and Operations
Medication Delivery US Region
Baxter Healthcare Corporation
Baxter, Aviva, Viaflex and Viaflo are trademarks of Baxter International Inc
Product Comparison Tables
| Differences | VIAFLEX (PVC) | AVIVA (non-PVC) | VIAFLO (non-PVC) |
| Product name presentation: “Injection, USP” vs “Intravenous Infusion BP” | 0.9% Sodium Chloride Injection, USP | 0.9% Sodium Chloride Injection, USP | Sodium Chloride 0.9% w/v Intravenous Infusion BP |
|
Content description |
Package insert and Container label: Each 100 mL contains 900 mg Sodium Chloride, USP |
Package insert and Container label: Each 100 mL contains 900 mg Sodium Chloride, USP |
Summary Product Characteristics: Sodium Chloride 9.0 g/L. Each mL contains 9 mg sodium chloride. Container Labels: Formula per 250 mL – Sodium Chloride 2.25 g Formula per 500 mL - Sodium Chloride 4.5 g Formula per 1000 mL – Sodium Chloride 9.0 g |
|
Content description |
Package insert and Container label: mEq per mL |
Package insert and Container label: mEq per mL |
Summary Product Characteristics: mmol/L* Container Labels: mmol per 250 mL mmol per 500 mL mmol per 1000 mL *1 mmol Sodium Chloride = 1 mEq Sodium Chloride |
| Storage conditions | 25°C/77°F | 25°C/77°F |
No storage statement present |
| Administration port closures | Pull off port protector (blue color) 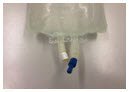 | Pull off port protector (natural/gum color)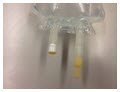 | Twist off port protector (white color)  |

Table 2. Comparison of Container Labels - 500 mL*
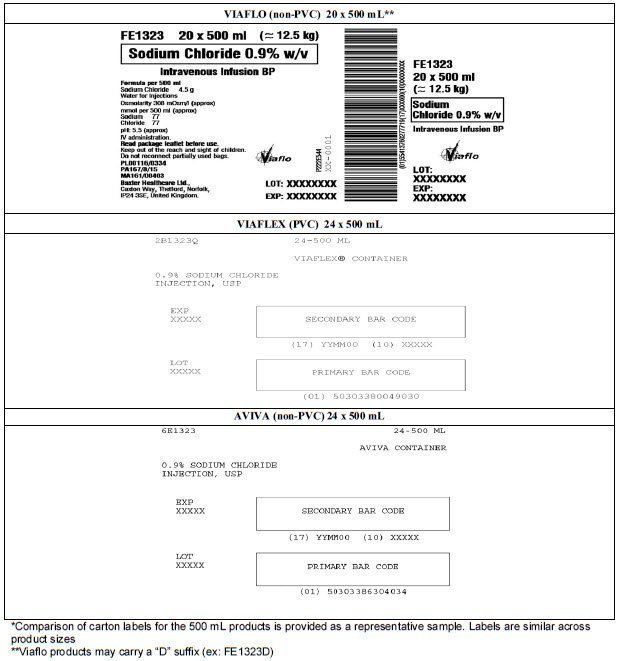
Table 3. Comparison of Carton Labels - 500 mL*
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
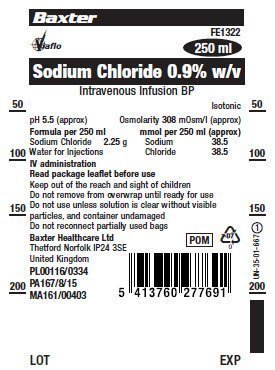
Container Label
Baxter Logo
Viaflo Logo
FE1322
250 ml
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
Isotonic
pH 5.5 (approx) Osmolarity 308 mOsm/l (approx)
Formula per 250 ml mmol per 250 ml (approx)
Sodium Chloride 2.25 g Sodium 38.5
Water for Injections Chloride 38.5
IV administration
Read package leaflet before use
Keep out of the reach and sight of children
Do not remove from overwrap until ready for use
Do not use unless solution is clear without visible
particles, and container undamaged
Do not reconnect partially used bags
Baxter Healthcare Ltd
Thetford Norfolk IP24 3SE
United Kingdom
PL00116/0334
PA167/8/15
MA161/00403
PDM
07
Bar Code
5413760277691
LOT
EXP
LN-35-01-667 1

Carton Label
FE1322
30 x 250 ml
(= 9.5 kg)
Sodium Chloride 0.9% w/v
Intravenous Infusion BP
Formula per 250 ml
Sodium Chloride 2.25 g
Water for Injections
Osmolarity 308 mOsm/l (approx)
mmol per 250 ml (approx)
Sodium 38.5
Chloride 38.5
pH: 5.5 (approx)
IV administration.
Read package leaflet before use.
Keep out of the reach and sight of children.
Do not reconnect partially used bags.
PL00116/0334
PA167/8/15
MA161/00403
Baxter Healthcare Ltd.,
Capton Way, Thetford, Norfolk,
IP24 3SE, United Kingdom.
Viaflo Logo
P111E547
XX-0001
LOT: XXXXXXXX
EXP: XXXXXXXX
Bar Code
(01)55413760277696(17)XXXX00(10)XXXXXXXX
FE1322
30 X 250 ML
(= 9.5 kg)
Sodium
Chloride 0.9% w/v
Intravenous Infusion BP
Viaflo Logo
LOT:
XXXXXXXX
EXP:
XXXXXXXX
| SODIUM CHLORIDE
sodium chloride injection, solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Registrant - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bieffe Medital SA | 464755693 | ANALYSIS(0338-9542) , MANUFACTURE(0338-9542) , LABEL(0338-9542) , PACK(0338-9542) , STERILIZE(0338-9542) | |
Biological Products Related to sodium chloride
Find detailed information on biosimilars for this medication.
Frequently asked questions
More about sodium chloride
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (3)
- Drug images
- Latest FDA alerts (19)
- Side effects
- Drug class: minerals and electrolytes
Patient resources
Professional resources
- Sodium Chloride monograph
- Sodium Chloride 20% Injection (AHFS Monograph)
- Bacteriostatic Sodium Chloride (FDA)
- Sodium Chloride 0.45% Injection (FDA)
- Sodium Chloride Inhalation Solution (FDA)

