Rezzayo: Package Insert / Prescribing Info
Package insert / product label
Generic name: rezafungin
Dosage form: injection, powder, lyophilized, for solution
Drug class: Echinocandins
J Code (medical billing code): J0349 (1 mg, injection)
Medically reviewed by Drugs.com. Last updated on Oct 29, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
REZZAYO™ (rezafungin for injection), for intravenous use
Initial U.S. Approval: 2023
Indications and Usage for Rezzayo
REZZAYO is an echinocandin antifungal indicated in patients 18 years of age or older who have limited or no alternative options for the treatment of candidemia and invasive candidiasis. Approval of this indication is based on limited clinical safety and efficacy data for REZZAYO. (1, 12.4, 14)
Limitations of Use
REZZAYO has not been studied in patients with endocarditis, osteomyelitis, and meningitis due to Candida. (1)
Rezzayo Dosage and Administration
- Administer the recommended dosage of REZZAYO once weekly by intravenous (IV) infusion, with an initial 400 mg loading dose, followed by a 200 mg dose once weekly thereafter. The safety of REZZAYO has not been established beyond 4 weekly doses. (2.1)
- See full prescribing information for reconstitution, dilution, and administration instructions. (2.2, 2.3, 2.4)
Dosage Forms and Strengths
For injection: 200 mg as a solid (cake or powder) in a single-dose vial for reconstitution. (3)
Contraindications
Known hypersensitivity to rezafungin or other echinocandins. (4)
Warnings and Precautions
- Infusion-related Reactions: REZZAYO may cause infusion-related reactions, including flushing, sensation of warmth, urticaria, nausea, or chest tightness. If these reactions occur, slow or pause the infusion. (5.1)
- Photosensitivity: REZZAYO may cause photosensitivity. Advise patients to use protection from sun exposure and other sources of UV radiation. (5.2)
- Hepatic Adverse Reactions: Abnormalities in liver tests have been seen in clinical trial patients treated with REZZAYO. Monitor patients who develop abnormal liver tests and evaluate patients for their risk/benefit of continuing REZZAYO therapy. (5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 5%) are hypokalemia, pyrexia, diarrhea, anemia, vomiting, nausea, hypomagnesemia, abdominal pain, constipation, and hypophosphatemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Melinta Therapeutics at 1-844-633-6568 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
Full Prescribing Information
1. Indications and Usage for Rezzayo
1.1 Indication
REZZAYO is indicated in patients 18 years of age or older who have limited or no alternative options for the treatment of candidemia and invasive candidiasis [see Microbiology (12.4)]. Approval of this indication is based on limited clinical safety and efficacy data for REZZAYO [see Clinical Studies (14)].
Limitations of Use
REZZAYO has not been studied in patients with endocarditis, osteomyelitis, and meningitis due to Candida.
1.2 Usage
Specimens for culture and other laboratory data (e.g., histopathology, non-culture diagnostics) should be obtained prior to initiating antifungal therapy. Therapy may be initiated before the results of the cultures and other laboratory tests are known. However, once these results become available, antifungal therapy should be adjusted accordingly.
2. Rezzayo Dosage and Administration
2.1 Recommended Dosage
Administer the recommended dosage of REZZAYO once weekly by intravenous (IV) infusion, with an initial 400 mg loading dose, followed by a 200 mg dose once weekly thereafter. The safety of REZZAYO has not been established beyond 4 weekly doses [see Adverse Reactions (6.1)]. REZZAYO is for intravenous infusion only [see Dosage and Administration (2.4)].
2.2 Missed Doses
If a scheduled dose is missed (not taken on the assigned day), administer the missed dose as soon as possible.
- If the missed dose is administered within 3 days of the assigned day, the next weekly dose may be given on schedule.
- If the missed dose is administered more than 3 days after the assigned day, revise the dosing schedule to ensure there are at least 4 days before the next dose.
- If restarting after at least 2 weeks of missed dosing, the dosing should be started again at the 400 mg loading dose.
2.3 Preparation and Administration of REZZAYO
Reconstitution
REZZAYO is supplied as a single-dose vial containing 200 mg of rezafungin.
For the 400 mg dose, aseptically reconstitute two vials each with 9.5 mL of sterile Water for Injection, to provide a concentration of 20 mg/mL in each vial.
For the 200 mg dose, aseptically reconstitute one vial with 9.5 mL of sterile Water for Injection, to provide a concentration of 20 mg/mL.
Swirl gently to dissolve the white to pale yellow cake or powder. Avoid shaking to minimize foaming. The solution should be clear to pale yellow after dissolution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the reconstituted solution is cloudy or has precipitated. The reconstituted solution is not for direct injection and must be diluted before intravenous infusion.
Storage of the Reconstituted Solution
REZZAYO reconstituted solution can be stored between 5°C to 25°C (41°F to 77°F). Stability of the reconstituted solution has been demonstrated for 24 hours when stored at 5°C to 25°C (41°F to 77°F).
Preparation of Intravenous Infusion Solution
See Table 1 for the dilution requirements for infusion solution. First, aseptically withdraw and discard the appropriate volume of diluent from the intravenous bag containing 250 mL of 0.9% Sodium Chloride Injection, 0.45% Sodium Chloride Injection, or 5% Dextrose Injection. Next, aseptically transfer the indicated volume of reconstituted solution (10 mL per vial) into the intravenous bag.
REZZAYO vials are single-dose vials. Discard any unused portion.
| Dose | Number of 200 mg Vials Required | Total Reconstituted Volume Required | Infusion Diluent Volume Discarded | Infusion Diluent Volume Used | Total Infusion Volume |
|---|---|---|---|---|---|
| 400 mg | 2 | 20 mL | 20 mL | 230 mL | 250 mL* |
| 200 mg | 1 | 10 mL | 10 mL | 240 mL | 250 mL† |
Storage of the Intravenous Infusion Solution
Store REZZAYO infusion solution between 5°C to 25°C (41°F to 77°F). Stability of the infusion solution has been demonstrated for 48 hours at 5°C to 25°C (41°F to 77°F).
The infusion solution must not be frozen.
2.4 Administration of Intravenous Infusion Solution
Administer REZZAYO by intravenous infusion over approximately one hour (~250 mL/h). If infusion-related reactions occur, the infusion may be slowed, or paused and restarted at a lower rate [see Warnings and Precautions (5.1)].
3. Dosage Forms and Strengths
For injection: 200 mg of rezafungin as a sterile white to pale yellow solid (cake or powder) for reconstitution in a single-dose glass vial.
4. Contraindications
REZZAYO is contraindicated in patients with known hypersensitivity to rezafungin or other echinocandins.
5. Warnings and Precautions
5.1 Infusion-Related Reactions
Infusion-related reactions, including flushing, sensation of warmth, urticaria, nausea, and chest tightness have been observed in clinical trials with REZZAYO. If these reactions occur, slow or pause the infusion and restart at a lower rate [see Dosage and Administration (2.4)].
5.2 Photosensitivity
REZZAYO may cause photosensitivity. Patients should be advised to use protection from sun exposure and other sources of UV radiation during REZZAYO treatment.
5.3 Hepatic Adverse Reactions
Abnormalities in liver tests have been seen in clinical trial patients treated with REZZAYO [see Adverse Reactions (6.1)]. In some patients with serious underlying medical conditions who were receiving multiple concomitant medications along with REZZAYO, clinically significant hepatic abnormalities have occurred. Monitor patients who develop abnormal liver tests during REZZAYO therapy and evaluate patients for their risk/benefit of continuing REZZAYO therapy.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Infusion-related Reactions [see Warnings and Precautions (5.1)]
- Hepatic Adverse Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of REZZAYO cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of REZZAYO was assessed in 76 subjects in phase 1 studies and 232 patients with candidemia and invasive candidiasis in Trials 1 and 2, who received a 400 mg loading dose followed by a 200 mg dose once weekly or higher (please note that after the loading dose of 400 mg, weekly doses higher than 200 mg are not approved). A total of 151 patients received an initial 400 mg loading dose followed by a 200 mg dose once weekly thereafter (400 mg/200 mg dose); the maximum duration of dosing was 4 weekly doses (including the loading dose).
In the pooled Trial 1 and 2 safety database of REZZAYO patients treated with the 400 mg/200 mg dose, the age range was 19-91 years, the gender distribution was 64.9% male and 35.1% females, and the race distribution was 66.2% White, 7.9% Black, 17.9% Asian, 2.7% other, and 5.3% not reported.
Adverse Reactions Leading to Discontinuation in Patients with Candidemia and Invasive Candidiasis
The number of patients with an adverse reaction leading to discontinuation of study medication was 9.3% in the REZZAYO arm and 9.0% in the caspofungin arm. In Trial 2, patients with a history (or presenting with significant symptoms) of severe ataxia, tremor, or neuropathy or a diagnosis of multiple sclerosis or a movement disorder (including Parkinson's Disease or Huntington's Disease) or currently taking a known neurotoxic medication were excluded from the trial.
Most Common Adverse Reactions in Patients with Candidemia and Invasive Candidiasis
Selected adverse reactions occurring in 5% or more of the patients, who received a 400 mg loading dose followed by a 200 mg dose of REZZAYO once weekly are shown in Table 2.
| Adverse Reaction | REZZAYO N = 151 n (%) | Caspofungin N = 166 n (%) |
|---|---|---|
| Gastrointestinal disorders | ||
| Diarrhea | 17 (11%) | 17 (10%) |
| Vomiting | 14 (9%) | 7 (4%) |
| Nausea | 13 (9%) | 8 (5%) |
| Abdominal pain | 11 (7%) | 9 (5%) |
| Constipation | 8 (5%) | 8 (5%) |
| Metabolism and nutrition disorders | ||
| Hypokalemia | 22 (15%) | 17 (10%) |
| Hypomagnesemia | 12 (8%) | 5 (3%) |
| Hypophosphatemia | 8 (5%) | 5 (3%) |
| General disorders | ||
| Pyrexia | 18 (12%) | 11 (7%) |
| Blood and lymphatic system disorders | ||
| Anemia | 15 (10%) | 13 (8%) |
Less Common Adverse Reactions in Patients with Candidemia and Invasive Candidiasis
The following selected adverse reactions occurred in <5% of patients receiving REZZAYO: infusion-related reactions, tremor, disseminated intravascular coagulation, dysphagia, gastrointestinal hemorrhage, fluid overload, insomnia, erythema, headache, dizziness, acute kidney injury, abnormal liver tests (including hypertransaminasemia and increased gamma-glutamyltransferase), peripheral neuropathy (includes neuropathy peripheral, polyneuropathy, and peroneal nerve palsy).
Tremors
Tremors were reported in 4/151 (2.6%) of REZZAYO-treated patients and none of the caspofungin-treated patients in Trials 1 and 2. All tremors developed in the second or third week after initiation of REZZAYO treatment and resolved within a month of onset.
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no data on the use of REZZAYO during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. No adverse embryofetal outcomes were observed when rezafungin was dosed intravenously to pregnant rats or rabbits during the period of organogenesis up to approximately 5 or 3 times the clinical exposure based on AUC comparison (see Data). In a pre- and post- natal study, there were no adverse effects on offspring growth, maturation, or measures of neurobehavioral or reproductive function in rats at doses up to about 5 times the recommended human dose based on AUC comparisons.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
In an embryofetal development study, intravenous rezafungin was administered at doses up to 45 mg/kg, once every 3 days to female rats one week prior to pairing with untreated males, and dosing was continued through mating to gestation day 17. Maternal toxicity included a transient histamine-release response (hypoactivity, ataxia, flushed extremities, dilated pupils and/or swollen facial area) at rezafungin doses of 15 mg/kg and above. No adverse embryofetal outcomes were observed in rat pups at rezafungin doses of 45 mg/kg, equivalent to 5 times the clinical exposure based on AUC comparisons.
No adverse outcomes were observed when rezafungin was dosed intravenously once every 3 days to pregnant rabbits during the period of organogenesis (GD 7 to 19) at doses up to 35 mg/kg (approximately 3 times the clinical exposure) despite maternal toxicity (reduced bodyweight gain).
In a pre- and post-natal study, there were no adverse effects on offspring growth, maturation, or measures of neurobehavioral or reproductive function in rats administered rezafungin intravenously once every 3 days from 1 week prior to mating through weaning (LD20), at doses up to 45 mg/kg/day (about 5 times the recommended human dose based on AUC comparisons).
8.2 Lactation
Risk Summary
There are no data on the presence of rezafungin or its metabolite in human milk, the effects on the breastfed infant, or the effects on milk production. Rezafungin was present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for REZZAYO and any potential adverse effects on the breastfed infant from REZZAYO or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Based on rat studies, rezafungin could lead to decreased sperm motility, decreased sperm numbers, and increased incidence of sperm with abnormal morphology. The effect of REZZAYO on human fertility is unknown [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of REZZAYO have not been established in pediatric patients.
8.5 Geriatric Use
Of the 151 rezafungin-treated patients at the proposed dose in Trials 1 and 2, 64 patients (42%) were 65 years of age and older, while 26 patients (17%) were 75 years of age and older.
Clinical studies of REZZAYO did not include sufficient numbers of older adult patients to determine if patients 65 years and older respond differently than younger adult patients.
10. Overdosage
No cases of overdose were reported during the clinical studies. Rezafungin is highly protein bound and not anticipated to be dialyzable.
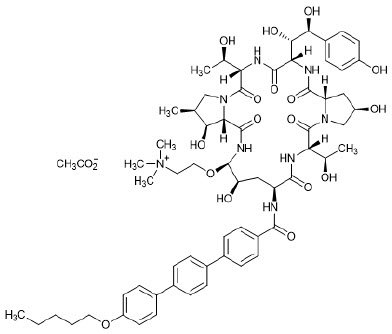
11. Rezzayo Description
REZZAYO (rezafungin for injection), for intravenous use is a sterile solid (cake or powder) that contains rezafungin acetate. Rezafungin acetate is a semisynthetic lipopeptide synthesized from a fermentation product of Aspergillus nidulans. REZZAYO is an echinocandin, a class of antifungal drugs that inhibits the synthesis of 1,3-β-D-glucan, an essential component of fungal cell walls.
REZZAYO contains 210 mg of rezafungin acetate, equivalent to 200 mg of rezafungin. REZZAYO also contains 47 mg histidine, 500 mg mannitol, 450 mg polysorbate 80, and hydrochloric acid and/or sodium hydroxide for pH adjustment. Rezafungin acetate is a hygroscopic, white to off-white powder. It is freely soluble in water, soluble in methanol, and sparingly soluble in ethanol.
Rezafungin acetate is chemically designated as Echinocandin B, 1-[(4R,5R)-4-hydroxy-N2-[[4"- (pentyloxy)[1,1':4',1"-terphenyl]-4-yl]carbonyl]-5-[2-(trimethylammonio)ethoxy]-L-ornithine]-4-[(4S)-4- hydroxy-4-(4-hydroxyphenyl)-L-allothreonine]-, acetate (1:1).
The empirical formula of rezafungin acetate is C63H85N8O17 • C2H3O2, and the formula weight is 1285.46 g/mol.
The chemical structure of rezafungin acetate is:
12. Rezzayo - Clinical Pharmacology
12.2 Pharmacodynamics
Rezafungin exposures achieved with the recommended dosage regimen appear to be on the plateau of the observed flat exposure-efficacy response curve in clinical studies.
Cardiac Electrophysiology
Rezafungin does not prolong the QTc interval to any clinically relevant extent at a dose 3.5 times the maximum approved recommended loading dose.
12.3 Pharmacokinetics
Following single and multiple dosing, the Cmax and AUC of rezafungin increase in a dose-proportional manner over a dose range of 50 mg (0.125 times the approved maximum recommended loading dose) to 400 mg.
Rezafungin population PK model derived pharmacokinetics (in patients with candidemia and invasive candidiasis (IC)) following administration of REZZAYO are provided in Table 3 and presented as mean ± standard deviation (SD). The mean rezafungin AUC0-168h decreased approximately 30% and Cmax decreased approximately 19% in patients with candidemia and IC compared to healthy participants.
| Parameter | Value* | |
|---|---|---|
| Exposure | Day 1 | Day 15 |
| Cmax(mcg/mL)† | 19.2 ± 5.9 | 11.8 ± 3.5 |
| AUC0-168 (mcg∙h/mL) | 827 ± 252 | 667 ± 224 |
| Cmin (mcg/mL) | 2.4 ± 0.9 | 2.2 ± 0.9 |
| Distribution | ||
| % Bound to human plasma proteins | Mean estimates varied from 87.5% to 93.6% in patients Mean estimates varied from 95.6% to >98.6% in healthy adults |
|
| Volume of distribution (V) | 67±28L | |
| Elimination | ||
| Clearance (CL) | 0.35 ± 0.13 L/hr | |
| terminal half-life (t1/2) | 152 ± 29 hours | |
| Metabolism | ||
| Metabolic pathways | Hepatic metabolism of rezafungin has not been observed. It is unlikely that rezafungin is a clinically relevant substrate of CYP450 enzymes | |
| Excretion‡ | ||
| Major route of elimination | Fecal excretion | |
| % feces | 74.3% of recovered radioactivity, primarily as rezafungin | |
| % urine | 25.7% of recovered radioactivity, primarily as inactive metabolites | |
| Cmax= maximum plasma concentration; Cmin= trough plasma concentration; AUC0–168h= area under the plasma concentration-time curve from time zero to 168 hours post dose | ||
Specific Populations
No clinically relevant effects on the pharmacokinetics of rezafungin were observed based on age (range: 20 to 89 years), sex (60.6% male), race (~10% Asian, 10% Black, 77% White), weight (range: 34 to 154.5 kg), or hepatic impairment (Child Pugh Class B or C). No clinically relevant effect on the pharmacokinetics of rezafungin were observed based on renal impairment (creatinine clearance: 9.3 mL/min to above 120 mL/min) and no effect is expected in patients undergoing hemodialysis.
Drug Interaction Studies
Clinical Studies
Drug-drug interaction studies' findings in healthy subjects show that at the recommended rezafungin dosing regimen, no clinically significant effect is expected of rezafungin treatment on the pharmacokinetics of substrates of cytochrome P450 (CYP) enzymes and/or drug transporters (repaglinide [CYP2C8], cyclosporine [CYP3A and P-gp], tacrolimus [CYP3A and P-gp], caffeine [CYP1A2], midazolam [CYP3A]), metformin [OCT-1 and OCT-2 and MATE-1 and MATE-2], pitavastatin [OATP], rosuvastatin [BCRP and OATP], digoxin [P-gp]). These studies also show no clinically significant effect expected of rezafungin treatment on the pharmacokinetics of other drugs likely to be co-administered (ibrutinib, venetoclax, efavirenz, or mycophenolate mofetil).
In Vitro Studies
Rezafungin is not a substrate of CYP enzymes or drug transporter systems. Rezafungin is not an inhibitor or inducer of common drug metabolizing CYP enzymes or transporter systems.
12.4 Microbiology
Mechanism of Action
Rezafungin is a semi-synthetic echinocandin. Rezafungin inhibits the 1,3-β-D-glucan synthase enzyme complex, which is present in fungal cell walls but not in mammalian cells. This results in inhibition of the formation of 1,3-β-D-glucan, an essential component of the fungal cell wall of many fungi, including Candida species (spp.). Inhibition of 1,3-β-D-glucan synthesis results in concentration-dependent in vitro fungicidal activity against Candida spp., however, the clinical significance of this activity is unknown.
Resistance
Reduced susceptibility to echinocandins predominantly arises from mutations in glucan synthase catalytic subunit-encoding FKS genes (FKS1 and/or FKS2) that impact residues comprising "hot spot" (HS) regions of the Fks protein. Rezafungin exhibits some degree of cross-resistance to all fks mutations that confer reduced susceptibility to echinocandins. The relevance of fks-based reduced susceptibility to clinical outcome has not been fully characterized for rezafungin.
Interaction with Other Antimicrobials
In vitro studies have not demonstrated antagonism between rezafungin and azoles, amphotericin B, or flucytosine. In vitro studies have not demonstrated antagonism for rezafungin versus bacteria in combination with the following antibacterial drug classes: aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, glycopeptides, macrolides, monobactams, oxazolidinones, polypeptides, rifamycins, sulfonamides, and tetracyclines, as well as daptomycin.
Antifungal Activity
Rezafungin has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections.
Candida albicans
Candida glabrata
Candida parapsilosis
Candida tropicalis
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following fungi exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for rezafungin against isolates of a similar genus or organism group. However, the efficacy of rezafungin in treating clinical infections caused by these fungi has not been established in adequate and well-controlled clinical trials.
Candida krusei
Candida auris
Candida dubliniensis
Candida fabianii
Candida guilliermondii
Candida inconspicua
Candida kefyr
Candida lusitaniae
Candida metapsilosis
Candida orthopsilosis
Candida pulcherrima
Candida rugosa
Candida sojae
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies with rezafungin have not been conducted.
Mutagenesis
Rezafungin was negative in a standard battery of assays, including an in vitro bacterial reverse mutation assay, an in vitro mammalian clastogenicity assay, and an in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
Rezafungin did not affect mating or fertility in male and female rats following IV administration once every 3 days at doses up to 45 mg/kg (6 times the clinical exposure, based on AUC determined in a separate rat study). Decreased sperm motility was noted at ≥30 mg/kg and most males at 45 mg/kg showed mild/moderate hypospermia and had no detectable motile sperm. At rezafungin doses ≥30 mg/kg there was an increased incidence of sperm with abnormal morphology as well as mild to moderate degeneration of the seminiferous tubules. In a 3-month study of every 3 day IV rezafungin in rats, males dosed at 45 mg/kg showed minimal tubular degeneration/atrophy in the testes and cellular debris in the epididymides at the end of 3 months. The incidence of this finding reduced by the end of a 4-week reversibility period. In contrast, sperm concentration, production rate, morphology and motility were unaffected in adult monkeys dosed weekly with rezafungin, up to 30 mg/kg (about 6 times the clinical dose based on AUC comparisons) for 11 or 22 weeks or after a 52-week recovery period.
13.2 Animal Toxicology and/or Pharmacology
In one 3-month study in monkeys using every-3-day dosing, tremors were observed, beginning 35 to 43 days after the beginning of dosing at and above 30 mg/kg (9 times the clinical exposure based on AUC comparison). Increased cellularity/hyperplasia of Schwann cells and nerve fiber degeneration (affecting axons and/or myelin) were observed at 30 mg/kg (9 times the clinical exposure based on AUC comparison) and above. Tremors and demyelination persisted in the fourth week of a 4-week reversibility period. A subsequent 26-week monkey study using weekly rezafungin dosing up to 30 mg/kg (6 times the clinical exposure based on AUC comparison) showed a non-dose dependent increase in tremors, axonal degeneration, or demyelination compared to controls beginning at doses similar to human exposures (5 mg/kg). In one animal in the 15 mg/kg cohort (3 times the clinical exposure based on AUC comparison), tremors persisted to the end of the 52-week reversibility period. In rats dosed weekly for 26 weeks, intravenous rezafungin was associated with an increased incidence of axonal/nerve fiber degeneration at 25 and 45 mg/kg (2- and 4-times the clinical dose based on AUC comparison) at the end of the 26-week reversibility period.
Rezafungin induced an acute transient histamine-release response in rats. In monkeys, both the vehicle and rezafungin induced a mild transient histamine-like response in some studies.
14. Clinical Studies
The safety and efficacy of REZZAYO in the treatment of patients with candidemia and/or invasive candidiasis (IC) were evaluated in a multicenter, randomized, double-blind study (Trial 1; NCT03667690). Patients were randomized in a 1:1 ratio to receive REZZAYO or caspofungin. Randomization was stratified based on diagnosis (candidemia only; IC) and by Acute Physiology and Chronic Health Evaluation II score (APACHE II)/absolute neutrophil count (ANC) at screening. Patients with septic arthritis in a prosthetic joint, osteomyelitis, endocarditis or myocarditis, meningitis, endophthalmitis, chorioretinitis, or any central nervous system infection, chronic disseminated candidiasis, or urinary tract candidiasis due to ascending Candida infection secondary to obstruction or surgical instrumentation of the urinary tract were excluded.
Patients in the REZZAYO arm were to receive a single 400 mg loading dose on Day 1 of Week 1, followed by 200 mg once weekly, for a total of two to four doses. Patients in the caspofungin arm were to receive a single 70 mg IV loading dose, followed by caspofungin 50 mg IV once daily treatment for a total of 2 to 4 weeks. After ≥3 days of IV therapy, patients in the caspofungin group could be switched to oral step-down therapy (fluconazole), if the patient met the criteria for cure and was preparing to be discharged.
One hundred and ninety-nine patients in the intent-to-treat (ITT) population were randomized. The age range was 19-91 years, the gender distribution was 62% male and 38% female, and the race distribution was 61% White, 5% Black, 29% Asian, and 5% other races or not reported. The median duration of therapy was 14 days in the two treatment arms.
The modified ITT (mITT) population included 187 patients with a culture positive for Candida species within 4 days before randomization and who received at least one dose of study drug. The most frequent species isolated at baseline was C. albicans (42%), followed by C. glabrata (26%), C. tropicalis (20%), and C. parapsilosis (13%). The majority (70%) of patients had a diagnosis of candidemia only. The majority (93%) of patients were not neutropenic (ANC ≥500) and 84% had APACHE II scores less than 20. Risk factors for candidemia were: receipt of broad-spectrum antibacterial drugs (71%), presence of a central venous catheter (60%), major surgery (35%), diabetes mellitus (29%), active malignancy (25%), and total parenteral nutrition (20%). Mechanically ventilated patients were 24% (17% and 30% in the REZZAYO and caspofungin group, respectively).
Efficacy was assessed by all-cause mortality at Day 30. The number and percentage of patients in each treatment group who were alive and deceased/unknown survival status at Day 30 was determined in the mITT population. Additional efficacy outcomes were global cure (mycological eradication/presumed eradication, clinical cure, and radiological cure [for patients with documented IC by radiologic or other imaging findings at baseline]), mycological eradication/presumed eradication, and investigator's assessment of clinical cure. Results of the efficacy endpoints are shown in Table 4.
| REZZAYO 400 mg/200 mg N = 93 n (%) | Caspofungin 70 mg/50 mg N = 94 n (%) | Difference (95% CI)* |
|
|---|---|---|---|
|
|||
| All-Cause Mortality (Day 30)† | 22 (23.7) | 20 (21.3) | 2.4 (-9.7, 14.4) |
| Global Cure‡ | |||
| Day 5 | 52 (55.9) | 49 (52.1) | 3.8 (-10.5, 17.9) |
| Day 14 | 55 (59.1) | 57 (60.6) | -1.5 (-15.4, 12.5) |
| Clinical Cure§ | |||
| Day 5 | 59 (63.4) | 70 (74.5) | -11.0 (-24.0, 2.3) |
| Day 14 | 62 (66.7) | 63 (67.0) | -0.4 (-13.8, 13.1) |
| Day 30 | 51 (54.8) | 52 (55.3) | -0.5 (-14.6, 13.7) |
| Mycological eradication/presumed eradication¶ | |||
| Day 5 | 64 (68.8) | 58 (61.7) | 7.1 (-6.6, 20.6) |
| Day 14 | 63 (67.7) | 62 (66.0) | 1.8 (-11.7, 15.2) |
A multicenter, randomized, dose-finding, exploratory, double-blind study was conducted in subjects with candidemia and/or invasive candidiasis (Trial 2: NCT02734862). The primary objectives of this study were to evaluate safety and tolerability of rezafungin and overall success (mycological eradication and resolution of systemic signs attributable to candidemia and/IC) at Day 14. The study provides safety and supportive efficacy data.
16. How is Rezzayo supplied
16.1 How Supplied
REZZAYO (rezafungin for injection) is supplied as sterile white to pale yellow solid (cake or powder) in a single-dose 20 mL Type I glass vial with a stopper, an aluminum seal, and blue polypropylene flip-off cap. The vial stopper is not made with natural rubber latex.
REZZAYO is available in the following packaging configuration:
One single-dose vial of REZZAYO 200 mg (NDC 70842-240-01)
16.2 Storage and Handling
REZZAYO Vials
REZZAYO vials should be stored at 20°C to 25°C (68°F to 77°F). Brief exposure to 15°C to 30°C (59°F to 86°F) permitted [see USP Controlled Room Temperature].
Reconstituted Solution
REZZAYO reconstituted solution can be stored between 5°C (41°F) and 25°C (77°F) for up to 24 hours [see Dosage and Administration (2.3)].
IV Infusion Solution
REZZAYO infusion solution can be stored between 5°C (41°F) and 25°C (77°F) for up to 48 hours. Do not freeze [see Dosage and Administration (2.3)].
17. Patient Counseling Information
Photosensitivity
Advise patients to use protection against sun exposure and other sources of UV radiation during treatment because REZZAYO may cause photosensitivity [see Warnings and Precautions (5.2)].
Distributed by:
Melinta Therapeutics LLC
Parsippany, NJ 07054
USA
Manufactured by:
Patheon Italia S.p.A., a Thermo Fisher Scientific company
Viale Gian Battista Stucchi 110
20900 Monza (MB)
Italy
MEL150-R000
Carton Label - One 200 mg Sterile Single-dose Vial - REZZAYO
PRINCIPAL DISPLAY PANEL
NDC 70842-240-01
Rx only
REZZAYO™
(rezafungin for injection)
200 mg per vial
*Vial contains rezafungin
200 mg (equivalent to 210 mg
of rezafungin acetate).
For intravenous Infusion Only
Must be reconstituted and
further diluted.
1 Sterile Single-Dose Vial
Discard unused portion.

| REZZAYO
rezafungin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Melinta Therapeutics, LLC (079949853) |
| Registrant - Mundipharma GmbH (315689034) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Italia S.p.A. | 338336589 | MANUFACTURE(70842-240) | |
More about Rezzayo (rezafungin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: echinocandins
- Breastfeeding
- En español