Revesta: Package Insert / Prescribing Info
Package insert / product label
Generic name: cholecalciferol
Dosage form: capsule
Drug class: Vitamins
Medically reviewed by Drugs.com. Last updated on Dec 9, 2024.
On This Page
Revesta Description
Revesta is an orally administered prescription Vitamin for the dietary management of patients with unique nutritional needs requiring increased folate levels, Vitamin D supplementation due to Vitamin D deficiency and other nutritional supplementation.
Revesta should be administered under the supervision of a licensed medical practitioner.
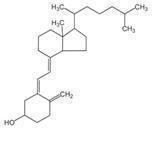
Vitamin D3 (cholecalciferol) is a white, crystalline powder, very soluble in water, with the following structural formula:
Each capsule contains:
Folic Acid: 1mg, Vitamin D3 (Cholecalciferol): 5750IU
Each capsule contains the following inactive ingredients: soybean oil, gelatin (bovine), yellow wax, glycerin, deionized water, lecithin, titanium dioxide, FD&C Blue #1.
Indications and Usage for Revesta
Revesta is indicated for dietary management of patients with unique nutritional needs requiring increased folate levels, Vitamin D deficiency or are in need of Vitamin D supplementation and other nutritional supplementation.
Revesta - Clinical Pharmacology
The in vivo synthesis of the major biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of ergocalciferol takes place in the liver (to 25-hydroxyvitamin D) and the second in the kidneys (to 1,25-dihydroxyvitamin D). Vitamin D metabolites promote the active absorption of calcium and phosphorus by the small intestine, thus elevating serum calcium and phosphate levels sufficiently to permit bone mineralization. Vitamin D metabolites also mobilize calcium and phosphate from bone and probably increase the reabsorption of calcium and perhaps also of phosphate by the renal tubules.
There is a time lag of 10 to 24 hours between the administration of vitamin D and the initiation of its action in the body due to the necessity of synthesis of the active metabolites in the liver and kidneys. Parathyroid hormone is responsible for the regulation of this metabolism in the kidneys.
Contraindications
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. Revesta is contraindicated in patients with hypercalcemia, malabsorption syndrome, abnormal sensitivity to the toxic effects of vitamin D, and hypervitaminosis D.
Warnings and Precautions
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor about the risks and benefits.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Adverse Reactions/Side Effects
This medication is generally well tolerated. Notify your doctor if you experience: nausea, loss of appetite, vomiting, stomach cramps, dry mouth, increased thirst, increased urination, muscle or bone pain,headache, weakness, weight loss, dizziness. If you notice other effects not listed above, contact your doctor or pharmacist.
How is Revesta supplied
Revesta capsules are supplied as blue capsules imprinted in white ink “330”, dispensed in HDPE plastic bottles of 30ct.
Storage and Handling
Store at controlled room temperature 15°-30°C (59°F-86°F). Keep in cool dry place.
Call your doctor about side effects. You may report side effects to FDA at 1-800-FDA-1088.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
OTHER SAFETY INFORMATION
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
Rx Only
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:Sterling Knight Pharmaceuticals, LLC
Ripley, MS 38663
Rev. 1114-1

| REVESTA
revesta capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sterling Knight Pharmaceuticals,LLC (079556942) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterling Knight Pharmaceuticals,LLC | 079556942 | manufacture(69336-330) , label(69336-330) | |
More about Revesta (cholecalciferol / folic acid)
- Check interactions
- Compare alternatives
- Imprints, shape & color data
- Side effects
- Dosage information
- Drug class: vitamins
