Primatene: Package Insert / Prescribing Info
Package insert / product label
Generic name: epinephrine
Dosage form: inhalant
Drug classes: Adrenergic bronchodilators, Catecholamines, Vasopressors
Medically reviewed by Drugs.com. Last updated on Jun 14, 2024.
Indications and Usage for Primatene
for temporary relief of mild symptoms of intermittent asthma
■ wheezing
■ tightness of chest
■ shortness of breath
Warnings
For oral inhalation only
Asthma alert: Because asthma may be life threatening, see a doctor if you
■ are not better in 20 minutes
■ get worse
■ need more than 8 inhalations in 24 hours
■ have more than 2 asthma attacks in a week
These may be signs that your asthma is
getting worse.
Do not use
■ unless a doctor said you have asthma
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs taken for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or a pharmacist before taking this product.
Ask a doctor before use if you have
■ ever been hospitalized for asthma
■ heart disease
■ high blood pressure
■ diabetes
■ trouble urinating due to an enlarged prostate gland
■ thyroid disease
■ seizures
■ narrow angle glaucoma
Ask a doctor or pharmacist before use if you are
■ taking prescription drugs for asthma, obesity, weight control, depression, or psychiatric or emotional conditions
■ taking any drug that contains phenylephrine, pseudoephedrine, ephedrine, or caffeine (such as for allergy, cough-cold, or pain)
When using this product
■ your blood pressure or heart rate may go up. This could increase your risk of heart attack or stroke, which may cause death.
■ your risk of heart attack or stroke increases if you:
■ have a history of high blood pressure or heart disease
■ take this product more frequently or take more than the recommended dose.
■ avoid foods or beverages that contain caffeine
■ avoid dietary supplements containing ingredients reported or claimed to have a stimulant effect
■ avoid spraying in eyes
■ contents under pressure. Do not puncture or incinerate.
■ do not store near open flame or heat above 120°F (49°C). May cause bursting.
Primatene Dosage and Administration
■ read the Consumer Information Insert for detailed directions on how to use this product
■ do not use more than directed
■ for adults and children 12 years of age and over
■ children under 12 years of age: do not use; it is not known if the drug works or is safe in children under 12
Before first use (new inhaler)
activate new inhaler by shaking then spraying into air 4 separate times
each time you dose
■ remove red cap (if attached)
■ shake then spray into the air 1 time
■ exhale completely, place inhaler in mouth
■ inhale deeply while pressing down on top of inhaler, then continue the deep breath
■ hold breath as long as possible, exhale
■ wait 1 minute. If symptoms not relieved, take a second inhalation by repeating steps above.
after use
■ wait at least 4 hours between doses
■ do not use more than 8 inhalations in 24 hours
■ wash inhaler after each day of use. Run water through the mouthpiece for 30 seconds.
Storage and Handling
■ store at room temperature, between 15-25°C (59-77°F)
■ contains no sulfites
■ keep this label and enclosed materials. They contain important additional information.
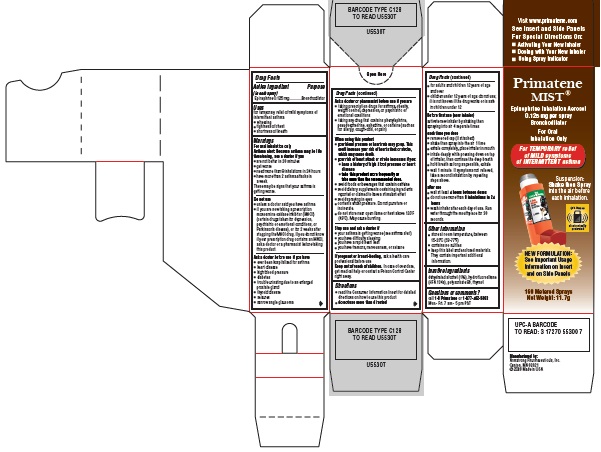
Primatene MIST® Carton
Principal Display Panel Text:
Primatene MIST®
Epinephrine Inhalation Aerosol
0.125 mg per spray
Bronchodilator
For Oral
Inhalation Only
For TEMPORARY relief
of MILD symptoms
of INTERMITTENT asthma
Suspension:
Shake then Spray
into the air before
each inhalation.
This item is
electronically
protected
NEW FORMULATION:
See Important Usage
Information on Insert
and on Side Panels
160 Metered Sprays
Net Weight: 11.7g
| PRIMATENE MIST
epinephrine inhalation aerosol |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Armstrong Pharmaceuticals, Inc. (809773794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Armstrong Pharmaceuticals, Inc. | 809773794 | manufacture(17270-5530) , analysis(17270-5530) | |
Frequently asked questions
- How is the new Primatene Mist different to the old formulation?
- Can you use an expired EpiPen in an emergency?
- Norepinephrine vs epinephrine: what's the difference?
- Can you bring an EpiPen on a plane?
- What's the mechanism of action for epinephrine?
- Does epinephrine cause vasoconstriction?
- How does neffy work?
- How much does Auvi-Q cost compared to EpiPen?
More about Primatene Mist (epinephrine)
- Check interactions
- Compare alternatives
- Reviews (42)
- Latest FDA alerts (3)
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: adrenergic bronchodilators
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
EpiPen, Adrenalin, neffy, Auvi-Q, ... +4 more
