Oxycodone and Acetaminophen Capsules: Package Insert / Prescribing Info
Package insert / product label
Generic name: oxycodone hydrochloride and acetaminophen
Dosage form: capsule, gelatin coated
Drug class: Narcotic analgesic combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
WARNING
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
Oxycodone and Acetaminophen Capsules Description
Each Oxycodone and Acetaminophen Capsule USP 5 mg/500 mg contains:
Oxycodone Hydrochloride USP..... 5 mg*
Acetaminophen USP..... 500 mg
*5 mg Oxycodone Hydrochloride USP is equivalent to 4.4815 mg oxycodone.
In addition, each capsule contains the following inactive ingredients: gelatin, magnesium stearate, silicon dioxide, sodium lauryl sulfate, pregelatinized starch, stearic acid, FD&C Blue No. 1, FD&C Red No. 40, FD&C Yellow No. 6, and titanium dioxide.
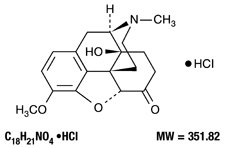
The oxycodone component is 4,5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride, a white, odorless, crystalline powder having a saline, bitter taste. It is derived from the opium alkaloid thebaine. Oxycodone hydrochloride may be represented by the structural formula shown below:
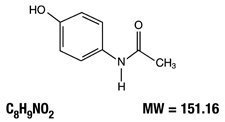
Acetaminophen, 4′-hydroxyacetanilide, is a non-opiate, non-salicylate analgesic and antipyretic which occurs as a white, odorless, crystalline powder with a slightly bitter taste. It has the following structural formula:
Oxycodone and Acetaminophen Capsules - Clinical Pharmacology
The principal ingredient, oxycodone, is a semisynthetic narcotic analgesic with multiple actions qualitatively similar to those of morphine; the most prominent of these involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value of the oxycodone in this product are analgesia and sedation.
Oxycodone is similar to codeine and methadone in that it retains at least one-half of its analgesic activity when administered orally.
Acetaminophen is a non-opiate, non-salicylate analgesic and antipyretic.
Indications and Usage for Oxycodone and Acetaminophen Capsules
Oxycodone and acetaminophen capsules are indicated for the relief of moderate to moderately severe pain.
Contraindications
This product should not be administered to patients who are hypersensitive to any components.
Hepatotoxicity
Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
Warnings
Rarely, acetaminophen may cause serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Patients should be informed about the signs of serious skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Hypersensitivity/anaphylaxis
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue oxycodone and acetaminophen capsules USP immediately and seek medical care if they experience these symptoms. Do not prescribe oxycodone and acetaminophen capsules USP for patients with acetaminophen allergy.
Drug Dependence
Oxycodone can produce drug dependence of the morphine type and, therefore, has the potential for being abused. Psychic dependence, physical dependence and tolerance may develop upon repeated administration of this drug, and it should be prescribed and administered with the same degree of caution appropriate to the use of other oral narcotic-containing medications. Like other narcotic-containing medications, oxycodone and acetaminophen capsules are subject to the Federal Controlled Substances Act (Schedule II).
Precautions
Head Injury and Increased Intracranial Pressure – The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions – The administration of products containing oxycodone or other narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Special Risk Patients – Oxycodone and acetaminophen capsules should be given with caution to certain patients such as the elderly or debilitated, and those with severe impairment of hepatic or renal function, hypothyroidism, Addison’s disease, and prostatic hypertrophy or urethral stricture.
Patient Counseling Information
- Do not take oxycodone and acetaminophen capsules USP if you are allergic to any of its ingredients.
- If you develop signs of allergy such as a rash or difficulty breathing stop taking oxycodone and acetaminophen capsules USP and contact your healthcare provider immediately.
- Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
Oxycodone may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient using oxycodone and acetaminophen capsules should be cautioned accordingly.
Drug Interactions
Patients receiving other narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with oxycodone and acetaminophen capsules may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
The concurrent use of anticholinergics with narcotics may produce paralytic ileus.
Pregnancy
Teratogenic Effects. Pregnancy Category C – Animal reproductive studies have not been conducted with oxycodone and acetaminophen capsules. It is also not known whether oxycodone and acetaminophen capsules can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Oxycodone and acetaminophen capsules should not be given to a pregnant woman unless in the judgment of the physician, the potential benefits outweigh the possible hazards.
Nonteratogenic Effects – Use of narcotics during pregnancy may produce physical dependence in the neonate.
Labor and Delivery
As with all narcotics, administration of oxycodone and acetaminophen capsules to the mother shortly before delivery may result in some degree of respiratory depression in the newborn and the mother, especially if higher doses are used.
Nursing Mothers
It is not known whether the components of this product are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when oxycodone and acetaminophen capsules are administered to a nursing woman.
Adverse Reactions/Side Effects
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea and vomiting. These effects seem to be more prominent in ambulatory than in non-ambulatory patients, and some of these adverse reactions may be alleviated if the patient lies down.
Other adverse reactions include allergic reactions, euphoria, dysphoria, constipation, skin rash and pruritus. At higher doses, oxycodone has most of the disadvantages of morphine including respiratory depression.
Drug Abuse and Dependence
Oxycodone and acetaminophen capsules are a Schedule II controlled substance.
Oxycodone can produce drug dependence and has the potential for being abused (see WARNINGS).
Overdosage
Following an acute overdosage, toxicity may result from the oxycodone or the acetaminophen.
Signs and Symptoms
Toxicity from oxycodone poisoning includes the opioid triad of: pinpoint pupils, depression of respiration, and loss of consciousness. Serious overdosage with oxycodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest, and death may occur.
In acetaminophen overdosage: dose-dependent potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and coagulation defects may also occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment
A single or multiple drug overdose with oxycodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered.
Oxycodone
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including oxycodone. Since the duration of action of oxycodone may exceed that of the antagonist, the patient should be kept under continued surveillance, and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. A narcotic antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Acetaminophen
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
Oxycodone and Acetaminophen Capsules Dosage and Administration
Dosage should be adjusted according to the severity of the pain and the response of the patient. However, it should be kept in mind that tolerance to oxycodone can develop with continued use and that the incidence of untoward effects is dose related. This product is inappropriate even in high doses for severe or intractable pain. Oxycodone and acetaminophen capsules are given orally.
The usual adult dosage is one capsule every 6 hours as needed for pain.
How is Oxycodone and Acetaminophen Capsules supplied
Each Oxycodone and Acetaminophen Capsule USP contains oxycodone hydrochloride 5 mg (equivalent to 4.4815 mg oxycodone) and acetaminophen 500 mg. It is available as a red/beige hard gelatin capsule imprinted with  532 identification number.
532 identification number.
- Bottles of 100……………NDC 0406-0532-01
- Bottles of 500……………NDC 0406-0532-05
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container with a child-resistant closure.
Protect from moisture.
DEA Order Form Required.
Mallinckrodt, the “M” brand mark, the Mallinckrodt Pharmaceuticals logo and M are trademarks of a Mallinckrodt company.
© 2013 Mallinckrodt.
Mallinckrodt Inc.
Hazelwood, MO 63042 USA
Printed in U.S.A.
Rev 10/2013
Mallinckrodt™
Pharmaceuticals
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 5 mg*/500 mg
Multiple strengths. Do not dispense unless strength is stated.
NDC 0406-0532-05
500 CAPSULES
OXYCODONE AND ACETAMINOPHEN CAPSULES USP
CII
5 mg*/500 mg
Each capsule contains:
Oxycodone Hydrochloride USP. . . . . . . 5 mg*
Acetaminophen USP. . . . . . . . . . . . . . 500 mg
Rx only
Mallinckrodt Pharmaceuticals™

| OXYCODONE AND ACETAMINOPHEN
oxycodone hydrochloride and acetaminophen capsule, gelatin coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Mallinckrodt, Inc. (047021092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MALLINCKRODT INC | 957414238 | ANALYSIS(0406-0532) , MANUFACTURE(0406-0532) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MALLINCKRODT, INC. | 097722284 | API MANUFACTURE(0406-0532) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MALLINCKRODT, INC. | 163205300 | API MANUFACTURE(0406-0532) | |
More about acetaminophen / oxycodone
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (876)
- Drug images
- Latest FDA alerts (5)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Drug class: narcotic analgesic combinations
- En español
Patient resources
Professional resources
- Oxycodone and Acetaminophen Oral Solution prescribing information
- Oxycodone and Acetaminophen Tablets (FDA)
Other brands
Percocet, Endocet, Roxicet, Tylox, ... +4 more