Odefsey: Package Insert / Prescribing Info
Package insert / product label
Generic name: emtricitabine, rilpivirine hydrochloride, and tenofovir alafenamide fumarate
Dosage form: tablet
Drug class: Antiviral combinations
Medically reviewed by Drugs.com. Last updated on Mar 9, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ODEFSEY® (emtricitabine, rilpivirine, and tenofovir alafenamide) tablets, for oral use
Initial U.S. Approval: 2016
WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of hepatitis B have been reported in patients with HIV-1 and HBV who have discontinued products containing emtricitabine (FTC) and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of ODEFSEY. Hepatic function should be monitored closely in these patients. If appropriate, anti-hepatitis B therapy may be warranted. (5.1)
Indications and Usage for Odefsey
ODEFSEY is a three-drug combination of emtricitabine (FTC) and tenofovir alafenamide (TAF), both HIV nucleoside analog reverse transcriptase inhibitors (NRTIs), and rilpivirine (RPV), a non-nucleoside reverse transcriptase inhibitor (NNRTI), and is indicated as a complete regimen for the treatment of HIV-1 infection in adult and pediatric patients weighing at least 25kg:
- as initial therapy in those with no antiretroviral treatment history with HIV-1 RNA less than or equal to 100,000 copies/mL; or
- to replace a stable antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies/mL) for at least 6 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of ODEFSEY. (1)
Limitations of Use:
Odefsey Dosage and Administration
- Testing: Prior to or when initiating ODEFSEY, test for hepatitis B virus infection. Prior to or when initiating ODEFSEY, and during treatment on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus (2.1)
- Recommended dosage: one tablet taken orally once daily with a meal. (2.2)
- For pregnant patients who are already on ODEFSEY prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL), one tablet taken once daily may be continued. Lower exposures of rilpivirine were observed during pregnancy, therefore viral load should be monitored closely. (2.3)
- Renal impairment: ODEFSEY is not recommended in patients with estimated creatinine clearance of 15 to below 30 mL per minute, or below 15 mL per minute who are not receiving chronic hemodialysis. (2.4)
Dosage Forms and Strengths
Tablets: 200 mg of FTC, 25 mg of RPV and 25 mg of TAF. (3)
Contraindications
ODEFSEY is contraindicated when coadministered with drugs where significant decreases in RPV plasma concentrations may occur, which may result in loss of virologic response and possible resistance and cross-resistance. (4)
Warnings and Precautions
- Skin and Hypersensitivity Reactions: Severe skin and hypersensitivity reactions have been reported during postmarketing experience with RPV-containing regimens, including cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Immediately discontinue treatment if hypersensitivity or rash with systemic symptoms or elevations in hepatic serum biochemistries develops and closely monitor clinical status, including hepatic serum biochemistries. (5.2)
- Hepatotoxicity: Hepatic adverse events have been reported in patients receiving an RPV-containing regimen. Monitor liver-associated tests before and during treatment with ODEFSEY in patients with underlying hepatic disease or marked elevations in liver-associated tests. Also consider monitoring liver-associated tests in patients without risk factors. (5.3)
- Depressive disorders: Severe depressive disorders have been reported. Immediate medical evaluation is recommended for severe depressive disorders. (5.4)
- New onset or worsening renal impairment: Assessment of serum creatinine, estimated creatinine clearance, urine glucose, and urine protein when initiating ODEFSEY and during therapy on a clinically appropriate schedule in all patients. Also assess serum phosphorus in patients with chronic kidney disease. (5.5)
- Concomitant use of ODEFSEY with drugs with a known risk to prolong the QTc interval of the electrocardiogram may increase the risk of Torsade de Pointes. (5.6)
- Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.7)
- Immune reconstitution syndrome: May necessitate further evaluation and treatment. (5.8)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence greater than or equal to 2%, all grades) are headache and sleep disturbances. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- ODEFSEY is a complete regimen for the treatment of HIV-1 infection; therefore, coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended. (7.1)
- Consult the Full Prescribing Information prior to and during treatment for important drug interactions. (4, 5.6, 7)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2025
Full Prescribing Information
WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B have been reported in patients with HIV-1 and HBV who have discontinued products containing emtricitabine (FTC) and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of ODEFSEY.
Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients with HIV-1 and HBV who discontinue ODEFSEY. If appropriate, anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
1. Indications and Usage for Odefsey
ODEFSEY is indicated as a complete regimen for the treatment of HIV-1 infection in adult and pediatric patients weighing at least 25 kg:
- as initial therapy in those with no antiretroviral treatment history with HIV-1 RNA less than or equal to 100,000 copies/mL or
- to replace a stable antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies/mL) for at least 6 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of ODEFSEY [see Microbiology (12.4) and Clinical Studies (14)].
Limitations of Use:
- More rilpivirine-treated participants with no antiretroviral treatment history with HIV-1 RNA greater than 100,000 copies/mL at the start of therapy experienced virologic failure (HIV-1 RNA ≥ 50 copies/mL) compared to rilpivirine-treated participants with HIV-1 RNA less than or equal to 100,000 copies/mL [see Clinical Studies (14.2,14.3)].
2. Odefsey Dosage and Administration
2.1 Testing Prior to Initiation and During Treatment with ODEFSEY
Prior to or when initiating ODEFSEY, test patients for hepatitis B virus infection [see Warnings and Precautions (5.1)].
Prior to or when initiating ODEFSEY, and during treatment with ODEFSEY, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.5)].
2.2 Recommended Dosage in Adult and Pediatric Patients Weighing at Least 25 kg
ODEFSEY is a three-drug fixed dose combination product containing 200 mg of emtricitabine (FTC), 25 mg of rilpivirine (RPV), and 25 mg of tenofovir alafenamide (TAF). The recommended dosage of ODEFSEY is one tablet taken orally once daily with a meal in adults and pediatric patients with body weight at least 25 kg and creatinine clearance greater than or equal to 30 mL per minute [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.3 Recommended Dosage During Pregnancy
For pregnant patients who are already on ODEFSEY prior to pregnancy and are virologically suppressed (HIV-1 RNA less than 50 copies per mL), one tablet of ODEFSEY taken once daily may be continued. Lower exposures of rilpivirine, a component of ODEFSEY, were observed during pregnancy, therefore viral load should be monitored closely [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.3)].
2.4 Not Recommended in Patients with Severe Renal Impairment
ODEFSEY is not recommended in patients with:
- severe renal impairment (estimated creatinine clearance of 15 to below 30 mL per minute); or
- end stage renal disease (ESRD; estimated creatinine clearance below 15 mL per minute) who are not receiving chronic hemodialysis [see Dosage and Administration (2.2), Warnings and Precautions (5.5), and Use in Specific Populations (8.6)].
3. Dosage Forms and Strengths
Each ODEFSEY tablet contains 200 mg of emtricitabine (FTC), 25 mg of rilpivirine (RPV) (equivalent to 27.5 mg of rilpivirine hydrochloride), and 25 mg of tenofovir alafenamide (TAF) (equivalent to 28 mg of tenofovir alafenamide fumarate).
The tablets are gray, capsule-shaped, film-coated and debossed with "GSI" on one side and "255" on the other side.
4. Contraindications
ODEFSEY is contraindicated when coadministered with the following drugs; coadministration may result in loss of virologic response and possible resistance to ODEFSEY or to the class of NNRTIs [see Warnings and Precautions (5.6), Drug Interactions (7) and Clinical Pharmacology (12.3)]:
- Anticonvulsants: carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- Antimycobacterials: rifampin, rifapentine
- Glucocorticoid (systemic): dexamethasone (more than a single-dose)
- Herbal Products: St. John's wort (Hypericum perforatum)
- Proton Pump Inhibitors: e.g., dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole
5. Warnings and Precautions
5.1 Severe Acute Exacerbation of Hepatitis B in Patients with HIV-1 and HBV
Test patients with HIV-1 for the presence of hepatitis B virus (HBV) before or when initiating antiretroviral therapy [see Dosage and Administration (2.1)].
Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients with HIV-1 and HBV who have discontinued products containing FTC and/or TDF, and may occur with discontinuation of ODEFSEY. Patients with HIV-1 and HBV who discontinue ODEFSEY should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment with ODEFSEY. If appropriate, anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since post treatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 Skin and Hypersensitivity Reactions
Severe skin and hypersensitivity reactions, including cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), have been reported during postmarketing experience with RPV-containing regimens. While some skin reactions were accompanied by constitutional symptoms such as fever, other skin reactions were associated with organ dysfunction, including elevations in hepatic serum biochemistries. During Phase 3 clinical trials of RPV, treatment-related rashes with at least Grade 2 severity were reported in 1% of participants. Overall, most rashes were Grade 1 or 2 and occurred in the first four to six weeks of therapy [see Adverse Reactions (6.2)].
Discontinue ODEFSEY immediately if signs or symptoms of severe skin or hypersensitivity reactions develop, including but not limited to, severe rash or rash accompanied by fever, blisters, mucosal involvement, conjunctivitis, facial edema, angioedema, hepatitis, or eosinophilia. Clinical status including laboratory parameters should be monitored and appropriate therapy should be initiated.
5.3 Hepatotoxicity
Hepatic adverse events have been reported in patients receiving an RPV-containing regimen. Patients with underlying hepatitis B or C virus infection, or marked elevations in liver-associated tests prior to treatment, may be at increased risk for worsening or development of liver-associated test elevations with use of ODEFSEY. A few cases of hepatic toxicity have been reported in adult patients receiving an RPV-containing regimen who had no preexisting hepatic disease or other identifiable risk factors. Appropriate laboratory testing prior to initiating therapy and monitoring for hepatotoxicity during therapy with ODEFSEY is recommended in patients with underlying hepatic disease such as hepatitis B or C, or in patients with marked elevations in liver-associated tests prior to treatment initiation. Liver-associated test monitoring should also be considered for patients without preexisting hepatic dysfunction or other risk factors.
5.4 Depressive Disorders
Depressive disorders (including depressed mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicidal ideation) have been reported with RPV. Promptly evaluate patients with severe depressive symptoms to assess whether the symptoms are related to ODEFSEY, and to determine whether the risks of continued therapy outweigh the benefits.
In Phase 3 trials of RPV in adult participants (N=1368) through 96 weeks, the incidence of depressive disorders (regardless of causality, severity) reported among RPV-treated participants (n=686) was 9%. Most events were mild or moderate in severity. In RPV-treated participants, the incidence of Grades 3 and 4 depressive disorders (regardless of causality) was 1%, the incidence of discontinuation due to depressive disorders was 1%, and suicidal ideation and suicide attempt was reported in 4 and 2 participants, respectively.
During the Phase 2 trial in RPV-treated pediatric participants 12 to less than 18 years of age (N=36), the incidence of depressive disorders (regardless of causality, severity) was 19% (7/36) through 48 weeks. Most events were mild or moderate in severity. The incidence of Grades 3 and 4 depressive disorders (regardless of causality) was 6% (2/36). None of the participants discontinued due to depressive disorders. Suicidal ideation and suicide attempt were reported in 1 participant.
5.5 New Onset or Worsening Renal Impairment
Postmarketing case of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome have been reported with TAF-containing products; while most of these cases were characterized by potential confounders that may have contributed to the reported renal events, it is also possible these factors may have predisposed patients to tenofovir-related adverse events [see Adverse Reactions (6.1, 6.2)]. ODEFSEY is not recommended in patients with estimated creatinine clearance of 15 to below 30 mL per minute, or in patients with estimated creatinine clearance below 15 mL per minute who are not receiving chronic hemodialysis.
Patients taking tenofovir prodrugs who have impaired renal function and those taking nephrotoxic agents, including nonsteroidal anti-inflammatory drugs are at increased risk of developing renal-related adverse reactions.
Prior to or when initiating ODEFSEY, and during treatment with ODEFSEY, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue ODEFSEY in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome.
5.6 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of ODEFSEY and other drugs may result in potentially significant drug interactions, some of which may lead to [see Contraindications (4), and Drug Interactions (7)]:
- Loss of therapeutic effect of ODEFSEY and possible development of resistance due to reduced exposure of RPV.
In healthy participants, higher than recommended doses of RPV (75 mg once daily and 300 mg once daily – 3 and 12 times the recommended dosages in ODEFSEY, respectively) have been shown to prolong the QTc interval of the electrocardiogram. Consider alternatives to ODEFSEY when coadministered with a drug that is known to have a risk of Torsade de Pointes [see Drug Interactions (7.2) and Clinical Pharmacology (12.2)].
See Table 3 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations [see Contraindications (4) and Drug Interactions (7)]. Consider the potential for drug interactions prior to and during ODEFSEY therapy and review concomitant medications during ODEFSEY therapy.
5.7 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including emtricitabine, a component of ODEFSEY, and tenofovir DF, another prodrug of tenofovir, alone or in combination with other antiretrovirals. Treatment with ODEFSEY should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.8 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including FTC and RPV, both components of ODEFSEY. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Severe Acute Exacerbations of Hepatitis B [see Warnings and Precautions (5.1)]
- Skin and Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
- Depressive Disorders [see Warnings and Precautions (5.4)]
- New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.5)]
- Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.7)]
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug (or a drug given in various combinations with other concomitant therapy) cannot be directly compared to rates in the clinical trials of another drug (or drug given in the same or different combination therapy) and may not reflect the rates observed in practice.
Adverse Reactions in Clinical Trials of ODEFSEY in Virologically-Suppressed Adult Participants with HIV-1
The safety of ODEFSEY in virologically-suppressed adults is based on Week 48 data from two randomized, double-blinded, active-controlled clinical trials, 1160 and 1216, that enrolled 1505 adult participants with HIV-1 who were virologically-suppressed for at least 6 months. Both trials were designed to compare switching to ODEFSEY to maintaining efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV/FTC/TDF) or emtricitabine/rilpivirine/tenofovir disoproxil fumarate (FTC/RPV/TDF) in Trials 1160 and 1216, respectively. A total of 754 participants received one tablet of ODEFSEY daily [see Clinical Studies (14.1)].
The most common adverse reactions (all Grades) reported in at least 2% of participants in the ODEFSEY group across Trials 1216 and 1160 were headache and sleep disturbances (Table 1). Over 98% of the adverse reactions in the ODEFSEY group were of mild to moderate intensity. The proportion of participants who discontinued treatment with ODEFSEY due to adverse events, regardless of severity, was 2% compared to 1% for FTC/RPV/TDF and 2% for EFV/FTC/TDF.
| Adverse Reaction | Trial 1160 | Trial 1216 | ||
|---|---|---|---|---|
| ODEFSEY (N=438) | EFV/FTC/TDF (N=437)† | ODEFSEY (N=316) | FTC/RPV/TDF (N=313)† |
|
| Headache | 2% | 1% | 0 | 1% |
| Sleep Disturbances | 2% | 1% | 0 | <1% |
| Flatulence | 1% | <1% | <1% | 1% |
| Abnormal Dreams | 1% | 1% | 0 | 2% |
| Diarrhea | 1% | 3% | 1% | 2% |
| Nausea | 1% | 1% | 1% | 1% |
Renal Laboratory Tests
In Trial 1216, the median baseline eGFR was 104 mL per minute for participants who switched to ODEFSEY from FTC/RPV/TDF (N=316) and the mean serum creatinine decreased by 0.02 mg per dL from baseline to Week 48.
In Trial 1160, the median baseline eGFR was 110 mL per minute for participants who switched to ODEFSEY from EFV/FTC/TDF (N=438), and the mean serum creatinine increased by 0.1 mg per dL from baseline to Week 48.
Bone Mineral Density Effects
Changes in BMD from baseline to Week 48 were assessed by dual-energy X-ray absorptiometry (DXA) in Trials 1216 and 1160.
In Trial 1216, mean bone mineral density (BMD) increased in participants who switched to ODEFSEY (1.61% lumbar spine, 1.04% total hip) and remained stable or decreased in participants who remained on FTC/RPV/TDF (0.08% lumbar spine, −0.25% total hip). BMD declines of 5% or greater at the lumbar spine were experienced by 1.7% of ODEFSEY participants and 3.0% of FTC/RPV/TDF participants. BMD declines of 7% or greater at the femoral neck were experienced by 0% of ODEFSEY participants and 1.2% of FTC/RPV/TDF participants.
In Trial 1160, mean BMD increased in participants who switched to ODEFSEY (1.65% lumbar spine, 1.28% total hip) and decreased slightly in participantswho remained on EFV/FTC/TDF (−0.05% lumbar spine, −0.13% total hip). BMD declines of 5% or greater at the lumbar spine were experienced by 2.3% of ODEFSEY participants and 4.9% of EFV/FTC/TDF participants. BMD declines of 7% or greater at the femoral neck were experienced by 1.4% of ODEFSEY participants and 3.3% of EFV/FTC/TDF participants. The long-term clinical significance of these BMD changes is not known.
Serum Lipids
Changes from baseline in total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, and total cholesterol to HDL ratio for Trials 1216 and 1160 are presented in Table 2.
| Trial 1216 | Trial 1160 | |||||||
|---|---|---|---|---|---|---|---|---|
| ODEFSEY N=316 [n=235] | FTC/RPV/TDF N=314 [n=245] | ODEFSEY N=438 [n=295] | EFV/FTC/TDF N=437 [n=308] |
|||||
| Baseline | Week 48 | Baseline | Week 48 | Baseline | Week 48 | Baseline | Week 48 | |
| mg/dL | Change*,† | mg/dL | Change*,† | mg/dL | Change*,† | mg/dL | Change*,† | |
|
||||||||
| Total Cholesterol (fasted) | 176 | +17 | 171 | 0 | 193 | -7 | 192 | -3 |
| HDL-Cholesterol (fasted) | 50 | +3 | 48 | 0 | 56 | -4 | 55 | -2 |
| LDL-Cholesterol (fasted) | 111 | +13 | 108 | +1 | 118‡ | -1‡ | 119 | -1 |
| Triglycerides (fasted) |
116 | +12 | 119 | -9 | 139 | -12 | 133 | +3 |
| Total Cholesterol to HDL Ratio | 3.7 | +0.2 | 3.8 | +0.1 | 3.7 | +0.2 | 3.8 | 0 |
Adverse Reactions in Clinical Trials of RPV-Containing Regimens in Treatment-Naïve Adult Participants with HIV-1
In pooled 96-week trials of antiretroviral treatment-naïve adult participants with HIV-1, the most common adverse reactions in participantstreated with RPV+FTC/TDF (N=550) (incidence greater than or equal to 2%, Grades 2−4) were headache, depressive disorders, and insomnia. The proportion of participants who discontinued treatment with RPV+FTC/TDF due to adverse reactions, regardless of severity, was 2%. The most common adverse reactions that led to discontinuation in this treatment group were psychiatric disorders (1.6%) and rash (0.2%). Although the safety profile was similar in virologically-suppressed adults with HIV-1 who were switched to RPV and other antiretroviral drugs, the frequency of adverse events increased by 20% (N=317).
Adrenal Function
In the pooled Phase 3 trials, at Week 96, there was an overall mean change from baseline in basal cortisol of -0.69 (-1.12, 0.27) micrograms/dL in the RPV group and of -0.02 (-0.48, 0.44) micrograms/dL in the EFV group.
In the RPV group, 43/588 (7%) of participants with a normal 250 micrograms ACTH stimulation test at baseline developed an abnormal 250 micrograms ACTH stimulation test (peak cortisol level <18.1 micrograms/dL) during the trial compared to 18/561 (3%) in the EFV group. Of the participants who developed an abnormal 250 micrograms ACTH stimulation test during the trial, 14 participants in the RPV group and 9 participants in the EFV group had an abnormal 250 micrograms ACTH stimulation test at Week 96. Overall, there were no serious adverse events, deaths, or treatment discontinuations that could clearly be attributed to adrenal insufficiency. The clinical significance of the higher abnormal rate of 250 micrograms ACTH stimulation tests in the RPV group is not known.
Adverse Reactions in Clinical Trials of FTC+TAF with EVG+COBI in Treatment-Naïve Adult Participants with HIV-1
In pooled 48-week trials of antiretroviral treatment-naïve adult participants with HIV-1, the most common adverse reaction in participants treated with FTC+TAF with EVG+COBI (N=866) (incidence greater than or equal to 10%, all grades) was nausea (10%). In this treatment group, 0.9% of participants discontinued FTC+TAF with EVG+COBI due to adverse event [see Clinical Studies (14)]. Antiretroviral treatment-naïve adult participants treated with FTC+TAF with EVG+COBI experienced mean increases of 30 mg/dL of total cholesterol, 15 mg/dL of LDL cholesterol, 7 mg/dL of HDL cholesterol and 29 mg/dL of triglycerides after 48 weeks of use.
Renal Laboratory Tests
In two 48-week trials in antiretroviral treatment-naïve adults with HIV-1 treated with FTC+TAF with elvitegravir (EVG) plus cobicistat (COBI) (N=866) with a median baseline eGFR of 115 mL per minute, mean serum creatinine increased by 0.1 mg per dL from baseline to Week 48.
In clinical trials of FTC+TAF with EVG+COBI in treatment-naïve participants and in virologically-suppressed participants switched to FTC+TAF with EVG+COBI with estimated creatinine clearance greater than 50 mL per minute, renal serious adverse events or discontinuations due to renal adverse reactions were encountered in less than 1% of participants treated with FTC+TAF with EVG+COBI.
In a 24-week trial in adults with renal impairment (baseline eGFR 30 to 69 mL per minute) who received FTC+TAF with EVG+COBI (N=248), mean serum creatinine was 1.5 mg per dL at both baseline and Week 24. FTC+TAF with EVG+COBI was permanently discontinued due to worsening renal function in two of 80 (3%) participants.
Bone Mineral Density Effects
In the pooled analysis of two 48-week trials of antiretroviral treatment-naïve adult participants with HIV-1, bone mineral density (BMD) from baseline to Week 48 was assessed by dual-energy X-ray absorptiometry (DXA). Mean BMD decreased from baseline to Week 48 by -1.30% with FTC+TAF with EVG+COBI at the lumbar spine and -0.66% at the total hip. BMD declines of 5% or greater at the lumbar spine were experienced by 10% of FTC+TAF with EVG+COBI participants. BMD declines of 7% or greater at the femoral neck were experienced by 7% of FTC+TAF with EVG+COBI participants. The long-term clinical significance of these BMD changes is not known.
Adverse Reactions in Clinical Trials in Pediatric Participants with HIV-1
In an open-label 48-week trial (TMC278-C213 Cohort 1) of 36 antiretroviral treatment-naïve pediatric participants with HIV-1 aged 12 to less than 18 years (weighing at least 32 kg) treated with 25 mg per day of RPV and other antiretrovirals, the most common adverse reactions were headache (19%), depression (19%), somnolence (14%), nausea (11%), dizziness (8%), abdominal pain (8%), vomiting (6%) and rash (6%).
In an open-label 48-week trial (TMC278-C213 Cohort 2) of 18 antiretroviral treatment-naïve pediatric participants with HIV-1 aged 6 to less than 12 years (weighing at least 17 kg) treated with RPV and other antiretrovirals, the most common adverse reactions were decreased appetite (17%), vomiting (11%), ALT increased (11%), AST increased (11%), and rash (11%).
In an open-label 48-week trial (TMC278HTX2002) of 26 virologically suppressed participants with HIV-1 less than 12 years of age (weighing at least 16 kg) treated with RPV and other antiretrovirals, the most commen adverse reactions were vomiting (15%), abdominal pain (12%), nausea (8%), ALT increased (12%), AST increased (8%), and decreased appetite (8%).
In a 48-week, open-label trial, 50 antiretroviral treatment-naïve pediatric participants with HIV-1 aged 12 to less than 18 years and weighing at least 35 kg (Cohort 1) and 52 virologically-suppressed pediatric participants aged 6 to less than 12 years and weighing at least 25 kg (Cohort 2) received FTC+TAF with EVG+COBI. With the exception of a decrease in the mean CD4+ cell count observed in Cohort 2 of this study, the safety profile in pediatric participants who received this combination was similar to that in adults.
Bone Mineral Density Effects
Among the pediatric participants in Cohort 1 receiving FTC+TAF with EVG+COBI, mean BMD increased from baseline to Week 48, +4.2% at the lumbar spine and +1.3% for the total body less head (TBLH). Mean changes from baseline BMD Z-scores were -0.07 for lumbar spine and -0.20 for TBLH at Week 48. In cohort 1, one participant had significant (at least 4%) lumbar spine BMD loss at Week 48.
Among the pediatric participants in Cohort 2 receiving FTC+TAF with EVG+COBI, mean BMD increased from baseline to Week 48, +3.9% at the lumbar spine and +4.2% for TBLH. Mean changes from baseline BMD Z-scores were -0.24 for lumbar spine and -0.19 for TBLH at Week 48. In Cohort 2, six participants had significant (at least 4%) lumbar spine BMD loss at Week 48; 2 participants also had at least 4% TBLH BMD loss at Week 48.
Change from Baseline in CD4+ Cell Counts
Although all participants in Cohort 2 receiving FTC+TAF with EVG+COBI had HIV-1 RNA < 50 copies/mL, there was a decrease from baseline in CD4+ cell count at Weeks 24 and 48. The mean baseline and mean change from baseline in CD4+ cell count and in CD4% from Week 2 to Week 48 are presented in Table 3. All participants maintained their CD4+ cell counts above 400 cells/mm3 [see Use in Specific Populations (8.4) and Clinical Studies (14.3)].
| Mean Change from Baseline | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 2 | Week 4 | Week 12 | Week 24 | Week 32 | Week 48 | |
|
|||||||
| CD4+ Cell Count (cells/mm3) | 961 (275.5)* | -117 | -114 | -112 | -118 | -62 | -66 |
| CD4% | 38 (6.4)* | +0.3% | -0.1% | -0.8% | -0.8% | -1.0% | -0.6% |
Adrenal Function in Clinical Trials of RPV in Pediatric Participants
In trial TMC278-C213 Cohort 1, at Week 48, the overall mean change from baseline in basal cortisol showed an increase of 1.59 (0.24, 2.93) micrograms/dL.
Six of 30 (20%) participants with a normal 250 micrograms ACTH stimulation test at baseline developed an abnormal 250 micrograms ACTH stimulation test (peak cortisol level <18.1 micrograms/dL) during the trial. Three of these participants had an abnormal 250 micrograms ACTH stimulation test at Week 48. Overall, there were no serious adverse events, deaths, or treatment discontinuations that could clearly be attributed to adrenal insufficiency. The clinical significance of the abnormal 250 micrograms ACTH stimulation tests is not known.
In trial TMC278-C213 Cohort 2, basal cortisol at baseline was normal (≥9 μg/dL) for 4/18 participants, low for 13/18 participants, and missing for 1/18 participants.
Among the 4 participants with normal basal cortisol at baseline, 3 participants had either normal basal cortisol levels (≥9 μg/dL) or normal cortisol levels 1 hour after ACTH stimulation (≥18.1 μg/dL) throughout the trial and/or at the last available visit (Week 24 and Week 72), and 1 participant had low basal cortisol at the last available assessment (Week 48) and no ACTH stimulation test was performed. Among the 13 participants with low basal cortisol pre-dose at baseline, 2 participants had low basal and ACTH stimulated cortisol values throughout the trial, including ACTH stimulated cortisol at baseline before starting treatment with RPV. For both participants, no adverse events suggestive for adrenal insufficiency were reported. The remaining 11 participants had normal serum cortisol values after ACTH stimulation at baseline and/or during treatment.
In trial TMC278HTX2002, 15/26 participants had either normal basal cortisol (≥9 μg/dL) or normal cortisol 1 hour after ACTH stimulation (≥18.1 μg/dL), 9 had low basal cortisol on Day 1, and in 2 participants the baseline value was missing.
From the 19 participants with low basal cortisol at Week 48, in 15 participants, the Week 48 serum cortisol levels returned to normal (≥248 nmol/L) after repeat serum basal cortisol testing or was normal after ACTH stimulation testing (≥500 nmol/L). In 4 participants, the serum cortisol levels remained low after repeat serum basal cortisol testing or after ACTH stimulation testing. At Week 48, 6 participants had normal (basal) cortisol (≥9 ug/dL) and the Week 48 result was not available for 1 participant.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing experience in patients receiving RPV or TAF-containing regimens. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
7.1 Not Recommended with Other Antiretroviral Medications
Because ODEFSEY is a complete regimen, coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended.
7.2 Drugs Inducing or Inhibiting CYP3A Enzymes
RPV is primarily metabolized by CYP3A, and drugs that induce or inhibit CYP3A may affect the clearance of RPV [see Clinical Pharmacology (12.3)]. Coadministration of RPV and drugs that induce CYP3A may result in decreased plasma concentrations of RPV and loss of virologic response and possible resistance to RPV or to the class of NNRTIs [see Contraindications (4), Warnings and Precautions (5.6), and Table 4].
Coadministration of RPV and drugs that inhibit CYP3A may result in increased plasma concentrations of RPV and possible adverse events.
7.3 Drugs Inducing or Inhibiting P-glycoprotein
TAF, a component of ODEFSEY, is a substrate of P-gp, BCRP, OATP1B1, and OATP1B3. Drugs that strongly affect P-gp and BCRP activity may lead to changes in TAF absorption (see Table 4). Drugs that induce P-gp activity are expected to decrease the absorption of TAF, resulting in decreased plasma concentration of TAF, which may lead to loss of therapeutic effect of ODEFSEY and development of resistance. Coadministration of ODEFSEY with other drugs that inhibit P-gp and BCRP may increase the absorption and plasma concentration of TAF.
7.4 Drugs Increasing Gastric pH
Coadministration of RPV with drugs that increase gastric pH may decrease plasma concentrations of RPV and lead to loss of virologic response and possible resistance to RPV or to the class of NNRTIs. Use of RPV with proton pump inhibitors is contraindicated and use of RPV with H2-receptor antagonists requires staggered administration [see Contraindications (4) and Table 4].
7.5 QT Prolonging Drugs
There is limited information available on the potential for a pharmacodynamic interaction between RPV and drugs that prolong the QTc interval. In a study of healthy participants, higher than recommended doses of RPV, 75 mg once daily and 300 mg once daily (3 times and 12 times recommended daily dose in ODEFSEY) prolonged the QTc interval [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.2)]. Consider alternative medications to ODEFSEY in patients taking a drug with a known risk of Torsade de Pointes.
7.6 Drugs Affecting Renal Function
Because FTC and tenofovir are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion, coadministration of ODEFSEY with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of FTC, tenofovir, and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.5)].
7.7 Significant Drug Interactions
Table 4 provides a listing of established or potentially clinically significant drug interactions with recommended steps to prevent or manage the drug interaction (the table is not all inclusive). The drug interactions described are based on studies conducted with either ODEFSEY, the components of ODEFSEY (FTC, RPV and TAF) as individual agents, or are predicted drug interactions that may occur with ODEFSEY [see Clinical Pharmacology (12.3), Tables 9–12]. For list of contraindicated drugs, [see Contraindications (4)].
| Concomitant Drug Class: Drug Name | Effect on Concentration† | Clinical Comment |
|---|---|---|
|
||
| Antacids:
antacids (e.g., aluminum, magnesium hydroxide, or calcium carbonate) | ↔ RPV (antacids taken at least 2 hours before or at least 4 hours after RPV) ↓ RPV (concomitant intake) | Administer antacids at least 2 hours before or at least 4 hours after ODEFSEY. |
| Anticonvulsants:
carbamazepine oxcarbazepine phenobarbital phenytoin | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| Antimycobacterials:
rifampin rifapentine | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| Antimycobacterials:
rifabutin | ↓ RPV‡
↓ TAF | Coadministration of ODEFSEY with rifabutin is not recommended. |
| Azole Antifungal Agents: fluconazole itraconazole ketoconazole posaconazole voriconazole | ↑ RPV‡,§
↑ TAF ↓ ketoconazole‡,§ | No dosage adjustment is required when ODEFSEY is coadministered with azole antifungal agents. Clinically monitor for breakthrough fungal infections when azole antifungals are coadministered with ODEFSEY. |
| Glucocorticoid (systemic):
dexamethasone (more than a single dose) | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| H2-Receptor Antagonists:
cimetidine famotidine nizatidine ranitidine | ↔ RPV‡,§
(famotidine taken 12 hours before RPV or 4 hours after RPV) ↓ RPV‡,§ (famotidine taken 2 hours before RPV) | Administer H2-receptor antagonists at least 12 hours before or at least 4 hours after ODEFSEY. |
| Herbal Products:
St. John's wort (Hypericum perforatum) | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
| Macrolide or Ketolide Antibiotics:
clarithromycin erythromycin telithromycin | ↑ RPV ↔ clarithromycin ↔ erythromycin ↔ telithromycin | Where possible, alternatives such as azithromycin should be considered. |
| Narcotic Analgesics:
methadone | ↓ R(–) methadone‡
↓ S(+) methadone‡ ↔ RPV‡ ↔ methadone‡ (when used with tenofovir) | No dosage adjustments are required when initiating coadministration of methadone with ODEFSEY. However, clinical monitoring is recommended, as methadone maintenance therapy may need to be adjusted in some patients. |
| Proton Pump Inhibitors:
e.g., dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole | ↓ RPV | Coadministration is contraindicated due to potential for loss of virologic response and development of resistance. |
7.8 Drugs Without Clinically Significant Interactions with ODEFSEY
Based on drug interaction studies conducted with the fixed dose combination or components of ODEFSEY, no clinically significant drug interactions have been observed when ODEFSEY is combined with the following drugs: acetaminophen, atorvastatin, chlorzoxazone, digoxin, ethinyl estradiol, ledipasvir, metformin, midazolam, norethindrone, norgestimate, sildenafil, simeprevir, sofosbuvir, velpatasvir, and voxilaprevir.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to ODEFSEY during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Available data from the APR show no statistically significant difference in the overall risk of major birth defects for emtricitabine (FTC), rilpivirine (RPV) or tenofovir alafenamide (TAF) compared with the background rate for major birth defects of 2.7% in a US reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) (see Data). The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in the clinically recognized pregnancies in the U.S. general population is 15–20%.
Based on the experience of pregnant individuals with HIV-1 who completed a clinical trial through the postpartum period with an RPV-based regimen, no dose adjustments are required for pregnant patients who are already on a stable RPV-containing regimen prior to pregnancy and who are virologically suppressed (HIV-1 RNA less than 50 copies per mL). Lower exposures of RPV were observed during pregnancy compared to the postpartum period. Therefore, viral load should be monitored closely [see Data and Clinical Pharmacology (12.3)].
In animal studies, no adverse developmental effects were observed when the components of ODEFSEY were administered separately during the period of organogenesis at exposures up to 60 and 108 times (mice and rabbits, respectively; FTC), 15 and 70 times (rats and rabbits, respectively; RPV) and equal to and 53 times (rats and rabbits, respectively; TAF) the exposure at the recommended daily dose of these components in ODEFSEY (see Data). Likewise, no adverse developmental effects were seen when FTC was administered to mice and RPV was administered to rats through lactation at exposures up to approximately 60 and 63 times, respectively, the exposure at the recommended daily dose of these components in ODEFSEY. No adverse effects were observed in the offspring when TDF was administered through lactation at tenofovir exposures of approximately 14 times the exposure at the recommended daily dosage of ODEFSEY.
Data
Human Data
Prospective reports from the APR of overall major birth defects in pregnancies exposed to drug components of ODEFSEY are compared with a U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP as the external comparator group. The MACDP population is not disease-specific, evaluates women and infants from a limited geographic area, and does not include outcomes for births that occurred at less than 20 weeks gestation.
Emtricitabine (FTC):
Based on prospective reports to the APR of over 5,400 exposures to FTC-containing regimens during pregnancy resulting in live births (including over 3,900 exposed in the first trimester and over 1,500 exposed in the second/third trimester), the prevalence of birth defects in live births was 2.6% (95% CI: 2.2% to 3.2%) and 2.7% (95% CI: 1.9% to 3.7%) following first and second/third trimester exposure, respectively, to FTC-containing regimens.
Rilpivirine (RPV):
RPV in combination with a background regimen was evaluated in a clinical trial of 19 pregnant participants with HIV-1 during the second and third trimesters and postpartum. Each of the subjects were on an RPV-based regimen at the time of enrollment. Twelve participants completed the trial through the postpartum period (6–12 weeks after delivery) and pregnancy outcomes are missing for six participants. The exposure (C0h and AUC) of total RPV was approximately 30 to 40% lower during pregnancy compared with postpartum (6 to 12 weeks). The protein binding of RPV was similar (>99%) during second trimester, third trimester, and postpartum period [see Clinical Pharmacology (12.3)]. One participant discontinued the trial following fetal death at 25 weeks gestation due to suspected premature rupture of membranes. Among the 12 participants who were virologically suppressed at baseline (less than 50 copies/mL), virologic response was preserved in 10 participants (83.3%) through the third trimester visit and in 9 participants (75%) through the 6–12 week postpartum visit. Virologic outcomes during the third trimester visit were missing for two participants who were withdrawn (one participant was nonadherent to the study drug and one participant withdrew consent). Among the 10 infants with HIV test results available, born to 10 pregnant participants with HIV-1, all had test results that were negative for HIV-1 at the time of delivery and up to 16 weeks postpartum. All 10 infants received antiretroviral prophylactic treatment with zidovudine. RPV was well tolerated during pregnancy and postpartum. There were no new safety findings compared with the known safety profile of RPV in adults with HIV-1.
Based on prospective reports to the APR of over 750 exposures to RPV-containing regimens during pregnancy resulting in live births (including over 550 exposed in the first trimester and over 200 exposed in the second/third trimester), the prevalence of birth defects in live births was 1.4% (95% CI: 0.6% to 2.8%) and 1.5% (95% CI: 0.3% to 4.3%) following first and second/third trimester exposure, respectively, to RPV-containing regimens.
Tenofovir Alafenamide (TAF):
Based on prospective reports to the APR of over 660 exposures to TAF-containing regimens during pregnancy resulting in live births (including over 520 exposed in the first trimester and over 130 exposed in the second/third trimester), the prevalence of birth defects in live births was 4.2% (95% CI: 2.6% to 6.3%) and 3.0% (95% CI: 0.8% to 7.5%) following first and second/third trimester exposure, respectively, to TAF-containing regimens.
Animal Data
Emtricitabine: FTC was administered orally to pregnant mice (250, 500, or 1000 mg/kg/day) and rabbits (100, 300, or 1000 mg/kg/day) through organogenesis (on gestation days 6 through 15, and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with FTC in mice at exposures (AUC) approximately 60 times higher and in rabbits at approximately 108 times higher than human exposures at the recommended daily dose. In a pre/postnatal development study with FTC, mice were administered doses up to 1000 mg/kg/day; no significant adverse effects directly related to drug were observed in the offspring exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60-fold higher than human exposures at the recommended daily dose.
Rilpivirine: RPV was administered orally to pregnant rats (40, 120, or 400 mg/kg/day) and rabbits (5, 10, or 20 mg/kg/day) through organogenesis (on gestation days 6 through 17, and 6 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with RPV in rats and rabbits at exposures 15 (rats) and 70 (rabbits) times higher than the exposure in humans at the recommended dose of 25 mg once daily. In a pre/postnatal development study with RPV, where rats were administered up to 400 mg/kg/day through lactation, no significant adverse effects directly related to drug were noted in the offspring.
Tenofovir Alafenamide: TAF was administered orally to pregnant rats (25, 100, or 250 mg/kg/day) and rabbits (10, 30, or 100 mg/kg/day) through organogenesis (on gestation days 6 through 17, and 7 through 20, respectively). No adverse embryo-fetal effects were observed in rats and rabbits at TAF exposures similar to (rats) and approximately 53 (rabbits) times higher than the exposure in humans at the recommended daily dose of ODEFSEY. TAF is rapidly converted to tenofovir; the observed tenofovir exposure in rats and rabbits were 59 (rats) and 93 (rabbits) times higher than human tenofovir exposures at the recommended daily doses. Since TAF is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice was observed after TAF administration compared to tenofovir disoproxil fumarate (TDF, another prodrug for tenofovir) administration, a pre/postnatal development study in rats was conducted only with TDF. Doses up to 600 mg/kg/day were administered through lactation, no adverse effects were observed in the offspring on gestation day 7 [and lactation day 20] at tenofovir exposures of approximately 14 [21] times higher than the exposures in humans at the recommended daily dose of ODEFSEY.
8.2 Lactation
Risk Summary
Data from the published literature report the presence of FTC, TAF, and TFV (tenofovir) in human milk; it is unknown if RPV is present in human milk. RPV is present in rat milk (see Data). Data from the published literature have not reported adverse effects of FTC or TAF on a breastfed child; it is not known if RPV has effects on the breastfed child. It is not known if the components of ODEFSEY affect milk production.
Potential risks of breastfeeding include: (1) HIV-1 transmission to infants without HIV-1, (2) developing viral resistance in infants with HIV-1, and (3) adverse reactions in a breastfed infant similar to those seen in adults.
8.4 Pediatric Use
The efficacy and safety of ODEFSEY as a complete regimen for the treatment of HIV-1 was established in pediatric patients 6 years of age and older with body weight greater than or equal to 25 kg [see Dosage and Administration (2.2)]. No pediatric clinical trials were conducted with ODEFSEY. Use of ODEFSEY in this age group is supported by adequate and well-controlled studies of RPV+FTC+TDF in adults with HIV-1 infection, adequate and well-controlled studies of FTC+TAF with EVG+COBI in adults with HIV-1, and by the following pediatric studies conducted using the components of ODEFSEY [see Clinical Studies (14)]:
- 48-week open-label trials of antiretroviral treatment-naïve pediatric participants with HIV-1 aged 12 to less than 18 years and weighing at least 32 kg (N=36) and aged 6 to less than 12 years weighing at least 17 kg (N=18) treated with RPV and other antiretrovirals. The safety and efficacy of RPV administered with other antiretrovirals were similar to that in antiretroviral treatment-naïve adults with HIV-1 on this regimen [see Adverse Reactions (6.1) and Clinical Studies (14)].
- 48-week open-label trial of 26 virologically-suppressed pediatric participants with HIV-1 aged less than 12 years old and weighing at least 16 kg (N=26) treated with RPV and other antiretrovirals. The safety and efficacy of RPV administered with other antiretrovirals were similar to that in virologically-suppressed adults with HIV-1 on this regimen [see Adverse Reactions (6.1)]
- 48-week open-label trials of antiretroviral treatment-naïve pediatric participants with HIV-1 aged 12 to less than 18 years and weighing at least 35 kg (N=50) and virologically-suppressed pediatric participants between the ages of 6 to less than 12 years weighing at least 25 kg (N=52) treated with FTC+TAF with EVG+COBI. The safety and efficacy of FTC+TAF with EVG+COBI were similar to that in adults with HIV-1 on this regimen, with the exception of a decrease from baseline in CD4+ cell counts in participants 6 to less than 12 years of age weighing at least 25 kg [see Adverse Reactions (6.1) and Clinical Studies (14)].
Because it is a fixed-dose combination tablet, the dose of ODEFSEY cannot be adjusted for patients of lower age and weight. The safety and efficacy of ODEFSEY have not been established in pediatric patients weighing less than 25 kg [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
8.5 Geriatric Use
In clinical trials, 80 of the 97 participants enrolled aged 65 years and over received FTC+TAF with EVG+COBI. No differences in safety or efficacy have been observed between elderly participants and those between 12 and less than 65 years of age. Clinical trials of RPV did not include sufficient numbers of participants aged 65 years and over to determine whether they respond differently from younger participants [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment of ODEFSEY is recommended in patients with estimated creatinine clearance greater than or equal to 30 mL per minute. ODEFSEY should be used with caution in adults patients with ESRD (estimated creatinine clearance below 15mL per minute) who are receiving chronic hemodialysis and increased monitoring is recommended for RPV-related adverse effects in patients with ESRD, as RPV concentrations may be increased due to alteration of drug absorption, distribution, and metabolism secondary to renal dysfunction. On days of hemodialysis, administer the daily dose of ODEFSEY after completion of hemodialysis treatment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
ODEFSEY is not recommended in patients with severe renal impairment (estimated creatinine clearance of 15 to below 30 mL per minute), or in patients with ESRD who are not receiving chronic hemodialysis, as the safety of ODEFSEY has not been established in these populations [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of ODEFSEY is recommended in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. ODEFSEY has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) [see Clinical Pharmacology (12.3)].
10. Overdosage
Limited data are available on overdose of the components of ODEFSEY in patients. If overdose occurs, monitor the patient for evidence of toxicity. Treatment of overdose with ODEFSEY consists of general supportive measures including monitoring of vital signs and ECG (QT interval) as well as observation of the clinical status of the patient.
Emtricitabine (FTC): Hemodialysis treatment removes approximately 30% of the FTC dose over a 3-hour dialysis period starting within 1.5 hours of FTC dosing (blood flow rate of 400 mL per minute and a dialysate flow rate of 600 mL per minute). It is not known whether FTC can be removed by peritoneal dialysis.
11. Odefsey Description
ODEFSEY (emtricitabine, rilpivirine, and tenofovir alafenamide) is a fixed-dose combination tablet containing emtricitabine (FTC), rilpivirine (RPV), and tenofovir alafenamide (TAF) for oral administration.
- FTC, a synthetic nucleoside analog of cytidine, is an HIV-1 nucleoside analog reverse transcriptase inhibitor (HIV-1 NRTI).
- RPV is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI).
- TAF, an HIV-1 NRTI, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
Each tablet contains 200 mg of FTC, 25 mg of RPV (equivalent to 27.5 of rilpivirine hydrochloride) and 25 mg of TAF (equivalent to 28 mg of tenofovir alafenamide fumarate) and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, and povidone. The tablets are film-coated with a coating material containing iron oxide black, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Emtricitabine: The chemical name of FTC is 4-amino-5-fluoro-1-(2R-hydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one. FTC is the (-)enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5 position.
FTC has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24 and has the following structural formula:
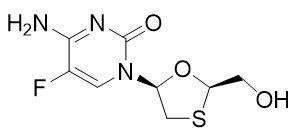
FTC is a white to off-white powder with a solubility of approximately 112 mg per mL in water at 25 °C.
Rilpivirine: The chemical name of rilpivirine hydrochloride drug substance is 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile monohydrochloride. Its molecular formula is C22H18N6 ∙ HCl and its molecular weight is 402.88. Rilpivirine hydrochloride has the following structural formula:
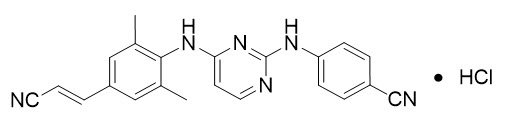
Rilpivirine hydrochloride is a white to almost white powder. Rilpivirine hydrochloride is practically insoluble in water over a wide pH range.
Tenofovir Alafenamide: The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1).
Tenofovir alafenamide fumarate has an empirical formula of C21H29O5N6P∙½(C4H4O4) and a formula weight of 534.50 and has the following structural formula:
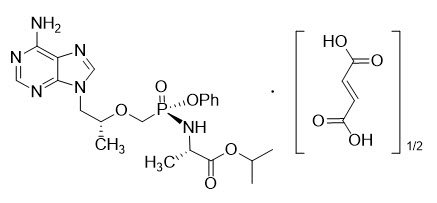
Tenofovir alafenamide fumarate is a white to off-white or tan powder with a solubility of 4.7 mg per mL in water at 20 °C.
12. Odefsey - Clinical Pharmacology
12.1 Mechanism of Action
ODEFSEY is a fixed dose combination of antiretroviral drugs emtricitabine, rilpivirine, and tenofovir alafenamide [see Microbiology (12.4)].
12.2 Pharmacodynamics
Cardiac Electrophysiology
When higher than recommended RPV doses of 75 mg (3 times the recommended dosage in ODEFSEY) once daily and 300 mg (12 times the recommended dosage in ODEFSEY) once daily were studied in healthy adults, the maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline correction were 10.7 (15.3) and 23.3 (28.4) milliseconds, respectively. Steady-state administration of RPV 75 mg once daily and 300 mg once daily resulted in a mean steady-state Cmax approximately 2.6 times and 6.7 times, respectively, higher than the mean Cmax observed with the recommended 25 mg once daily dose of RPV [see Warnings and Precautions (5.6)].
The effect of RPV at the recommended dose of 25 mg once daily on the QTcF interval was evaluated in a randomized, placebo-, and active- (moxifloxacin 400 mg once daily) controlled crossover study in 60 healthy adults, with 13 measurements over 24 hours at steady state. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline correction was 2 (5) milliseconds (i.e., below the threshold of clinical concern).
In a thorough QT/QTc study in 48 healthy participants, TAF at the recommended dose and at a dose approximately 5 times the recommended dose, did not affect the QT/QTc interval and did not prolong the PR interval.
The effect of FTC on the QT interval is not known.
12.3 Pharmacokinetics
Absorption, Distribution, Metabolism, and Excretion
The pharmacokinetic properties of the components of ODEFSEY are provided in Table 5. The multiple dose pharmacokinetic parameters of FTC, RPV and TAF and its metabolite tenofovir are provided in Table 6.
| Rilpivirine | Emtricitabine | Tenofovir Alafenamide | |
|---|---|---|---|
| PBMCs = peripheral blood mononuclear cells; CES1 = carboxylesterase 1. | |||
|
|||
| Absorption | |||
| Tmax (h) | 4 | 3 | 1 |
| Effect of moderate fat meal (relative to fasting)* | AUC Ratio = 1.13 (1.03, 1.23) | AUC Ratio = 0.91 (0.89, 0.93) | AUC Ratio = 1.45 (1.33, 1.58) |
| Effect of high fat meal (relative to fasting)* | AUC Ratio = 1.72 (1.49, 1.99) | AUC Ratio = 0.88 (0.85, 0.90) | AUC Ratio = 1.53 (1.39, 1.69) |
| Distribution | |||
| % Bound to human plasma proteins | ~99 | <4 | ~80 |
| Source of protein binding data | In vitro | In vitro | Ex vivo |
| Blood-to-plasma ratio | 0.7 | 0.6 | 1.0 |
| Metabolism | |||
| Metabolism | CYP3A | Not significantly metabolized | Cathepsin A† (PBMCs) CES1 (hepatocytes) CYP3A (minimal) |
| Elimination | |||
| Major route of elimination | Metabolism | Glomerular filtration and active tubular secretion | Metabolism (>80% of oral dose) |
| t1/2 (h)‡ | 50 | 10 | 0.51 |
| % Of dose excreted in urine§ | 6 | 70 | <1 |
| % Of dose excreted in feces§ | 85 | 13.7 | 31.7 |
| Parameter Mean (CV%) | Emtricitabine* | Rilpivirine† | Tenofovir Alafenamide‡ | Tenofovir§ |
|---|---|---|---|---|
| CV = Coefficient of Variation; NA = Not Applicable | ||||
|
||||
| Cmax
(microgram per mL) | 2.1 (20.2) | NA | 0.16 (51.1) | 0.02 (26.1) |
| AUCtau
(microgram∙hour per mL) | 11.7 (16.6) | 2.2 (38.1) | 0.21 (71.8) | 0.29 (27.4) |
| Ctrough
(microgram per mL) | 0.10 (46.7) | 0.08 (44.3) | NA | 0.01 (28.5) |
Specific Populations
Geriatric Patients
The pharmacokinetics of FTC and TAF have not been fully evaluated in the elderly (65 years of age and older). Population pharmacokinetics analysis of participants with HIV-1 in Phase 2 and Phase 3 trials of FTC+TAF with EVG+COBI showed that age did not have a clinically relevant effect on exposures of TAF up to 75 years of age.
The pharmacokinetics of RPV have not been fully evaluated in the elderly (65 years of age and older) [see Use in Specific Populations (8.5)].
Pediatric Patients
Exposures of TAF in 24 pediatric participants with HIV-1 aged 12 to less than 18 years who received FTC+TAF with EVG+COBI were decreased (23% for TAF AUC) compared to exposures achieved in treatment-naïve adults following administration of FTC+TAF with EVG+COBI. These exposure differences are not thought to be clinically significant based on exposure-response relationships. FTC exposures were similar in adolescents compared to treatment-naïve adults.
Exposures of FTC, TAF and TFV in 23 pediatric participants with HIV-1 aged 6 to less than 12 years who received FTC+TAF with EVG+COBI were higher (50 to 80% for AUC) than exposures achieved in adults following the administration of FTC+TAF with EVG+COBI, however, the increase was not considered clinically significant as the safety profiles were similar in adult and pediatric participants.
The PK of RPV in pediatric participants with HIV aged 6 to less than 18 years who received RPV 25 mg once daily were comparable to or slightly higher than those obtained in adults with HIV-1 [see Use In Specific Populations (8.4)].
Race and Gender
No clinically significant changes in the pharmacokinetics of the components of ODEFSEY have been observed based on race or gender.
Patients with Renal Impairment
Rilpivirine: Population pharmacokinetic analysis indicated that RPV exposure was similar in participants with HIV-1 and eGFR 60 to 89 mL per minute by Cockcroft-Gault method, relative to participants with HIV-1 and normal renal function. There is limited or no information regarding the pharmacokinetics of RPV in patients with moderate or severe renal impairment or in patients with end-stage renal disease [see Use in Specific Populations (8.6)].
Emtricitabine and Tenofovir Alafenamide: The pharmacokinetics of FTC+TAF with EVG+COBI in participants with HIV-1 and renal impairment (eGFR 30 to 69 mL per minute by Cockcroft-Gault method), and in participants with HIV-1 and ESRD (estimated creatinine clearance of less than 15 mL per minute by Cockcroft-Gault method) receiving chronic hemodialysis were evaluated in subsets of virologically-suppressed participants in open-label trials. The pharmacokinetics of TAF were similar among healthy participants, participants with HIV-1 and mild or moderate renal impairment, and participants with HIV-1 and ESRD receiving chronic hemodialysis; increases in FTC and TFV exposures in participants with HIV-1 and renal impairment were not considered clinically relevant (Table 7).
| AUCtau (microgram-hour per mL) Mean (CV%) |
||||
|---|---|---|---|---|
| Estimated Creatinine Clearance* | ≥90 mL per minute (N=18)† | 60–89 mL per minute (N=11)‡ | 30–59 mL per minute (N=18)§ | <15 mL per minute (N=12)¶ |
|
||||
| Emtricitabine | 11.4 (11.9) | 17.6 (18.2) | 23.0 (23.6) | 62.9 (48.0)# |
| Tenofovir | 0.32 (14.9) | 0.46 (31.5) | 0.61 (28.4) | 8.72 (39.4)Þ |
Patients with Hepatic Impairment
Emtricitabine: The pharmacokinetics of FTC have not been studied in participants with hepatic impairment; however, FTC is not significantly metabolized by liver enzymes, so the impact of hepatic impairment should be limited.
Rilpivirine: In a study comparing 8 participants with mild hepatic impairment (Child-Pugh score A) to 8 matched controls and 8 participants with moderate hepatic impairment (Child-Pugh score B) to 8 matched controls, the multiple-dose exposure of RPV was 47% higher in participants with mild hepatic impairment and 5% higher in participants with moderate hepatic impairment [see Use in Specific Populations (8.7)].
Tenofovir Alafenamide: Clinically relevant changes in the pharmacokinetics of tenofovir alafenamide or its metabolite tenofovir were not observed in participants with mild, moderate (Child-Pugh A and B), or severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.7)].
Hepatitis B and/or Hepatitis C Virus Coinfection
The pharmacokinetics of FTC and TAF have not been fully evaluated in participants with hepatitis B and/or C virus. Population pharmacokinetic analysis indicated that hepatitis B and/or C virus coinfection had no clinically relevant effect on the exposure of RPV.
Pregnancy and Postpartum
Rilpivirine: The exposure (C0h and AUC24h) to total RPV after intake of RPV 25 mg once daily as part of an antiretroviral regimen was 30 to 40% lower during pregnancy (similar for the second and third trimester), compared with postpartum (see Table 8). However, the exposure during pregnancy was not significantly different from exposures obtained in Phase 3 trials of RPV-containing regimens. Based on the exposure-response relationship for RPV, this decrease is not considered clinically relevant in patients who are virologically-suppressed. The protein binding of RPV was similar (>99%) during the second trimester, third trimester, and postpartum.
| Pharmacokinetics of total rilpivirine (mean ± SD, tmax: median [range]) | Postpartum (6–12 Weeks) (n=11) | 2nd Trimester of pregnancy (n=15) | 3rd Trimester of pregnancy (n=13) |
|---|---|---|---|
| C0h, ng/mL | 111 ± 69.2 | 65.0 ± 23.9 | 63.5 ± 26.2 |
| Cmin, ng/mL | 84.0 ± 58.8 | 54.3 ± 25.8 | 52.9 ± 24.4 |
| Cmax, ng/mL | 167 ± 101 | 121 ± 45.9 | 123 ± 47.5 |
| tmax, h | 4.00 (2.03–25.08) | 4.00 (1.00–9.00) | 4.00 (2.00–24.93) |
| AUC24h, ng.h/mL | 2714 ± 1535 | 1792 ± 711 | 1762 ± 662 |
Drug Interaction Studies
Rilpivirine: RPV is primarily metabolized by CYP3A, and drugs that induce or inhibit CYP3A may thus affect the clearance of RPV.
RPV at a dose of 25 mg once daily is not likely to have a clinically relevant effect on the exposure of medicinal products metabolized by CYP enzymes.
TAF is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or UGT1A1. TAF is a weak inhibitor of CYP3A in vitro. TAF is not an inhibitor or inducer of CYP3A in vivo.
The drug interaction studies described in Tables 9–12 were conducted with ODEFSEY (FTC/RPV/TAF) or the components of ODEFSEY (FTC, RPV, or TAF) administered individually.
The effects of coadministered drugs on the exposures of RPV and TAF are shown in Tables 9 and 10, respectively. The effects of RPV and TAF on the exposure of coadministered drugs are shown in Tables 11 and 12, respectively. For information regarding clinical recommendations, see Drug Interactions (7).
| Coadministered Drug | Dose/Schedule | Mean Ratio of RPV Pharmacokinetic Parameters With/Without Coadministered Drug (90% CI); No Effect = 1.00 |
||||
|---|---|---|---|---|---|---|
| Coadministered Drug (mg) | RPV (mg) | N | Cmax | AUC | Cmin | |
| CI=Confidence Interval; N=maximum number of participants with data; NA=Not Available; ↔=no change | ||||||
|
||||||
| Acetaminophen | 500 single dose | 150 once daily* | 16 | 1.09 (1.01, 1.18) | 1.16 (1.10, 1.22) | 1.26 (1.16, 1.38) |
| Atorvastatin | 40 once daily | 150 once daily* | 16 | 0.91 (0.79, 1.06) | 0.90 (0.81, 0.99) | 0.90 (0.84, 0.96) |
| Chlorzoxazone | 500 single dose taken 2 hours after RPV | 150 once daily* | 16 | 1.17 (1.08, 1.27) | 1.25 (1.16, 1.35) | 1.18 (1.09, 1.28) |
| Ethinylestradiol/Norethindrone | 0.035 once daily /1 mg once daily | 25 once daily† | 15 | ↔‡ | ↔‡ | ↔‡ |
| Famotidine | 40 single dose taken 12 hours before RPV | 150 single dose* | 24 | 0.99 (0.84, 1.16) | 0.91 (0.78, 1.07) | NA |
| Famotidine | 40 single dose taken 2 hours before RPV | 150 single dose* | 23 | 0.15 (0.12, 0.19) | 0.24 (0.20, 0.28) | NA |
| Famotidine | 40 single dose taken 4 hours after RPV | 150 single dose* | 24 | 1.21 (1.06, 1.39) | 1.13 (1.01, 1.27) | NA |
| Ketoconazole | 400 once daily | 150 once daily* | 15 | 1.30 (1.13, 1.48) | 1.49 (1.31, 1.70) | 1.76 (1.57, 1.97) |
| Methadone | 60–100 once daily, individualized dose | 25 once daily† | 12 | ↔‡ | ↔‡ | ↔‡ |
| Ledipasvir/Sofosbuvir | 90/400 once daily | 25 once daily§ | 42 | 0.97 (0.92, 1.02) | 0.95 (0.91, 0.98) | 0.93 (0.89, 0.97) |
| Omeprazole | 20 once daily | 25 single dose† | 15 | 0.30 (0.24, 0.38) | 0.35 (0.28, 0.44) | NA |
| Rifabutin | 300 once daily | 25 once daily† | 18 | 0.69 (0.62, 0.76) | 0.58 (0.52, 0.65) | 0.52 (0.46, 0.59) |
| Rifampin | 600 once daily | 150 once daily* | 16 | 0.31 (0.27, 0.36) | 0.20 (0.18, 0.23) | 0.11 (0.10, 0.13) |
| Simeprevir | 25 once daily | 150 once daily† | 23 | 1.04 (0.95, 1.30) | 1.12 (1.05, 1.19) | 1.25 (1.16, 1.35) |
| Sildenafil | 50 single dose | 75 once daily* | 16 | 0.92 (0.85, 0.99) | 0.98 (0.92, 1.05) | 1.04 (0.98, 1.09) |
| Sofosbuvir/velpatasvir | 400/100 once daily | 10 once daily¶ | 24 | 0.93 (0.88,0.98) | 0.95 (0.90, 1.00) | 0.96 (0.90,1.03) |
| Sofosbuvir/velpatasvir/voxilaprevir | 400/100/100 + 100 voxilaprevir# once daily | 25 once daily§ | 30 | 0.79 (0.74, 0.84) | 0.80 (0.76, 0.85) | 0.82 (0.77, 0.87) |
| Coadministered Drug | Dose of Coadministered Drug (mg) | TAF (mg) | N | Mean Ratio of Tenofovir Alafenamide Pharmacokinetic Parameters (90% CI); No Effect = 1.00 |
||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||
| CI=Confidence Interval; N=maximum number of participants with data; NA=Not Available | ||||||
| Cobicistat* | 150 once daily | 8 once daily | 12 | 2.83 (2.20, 3.65) | 2.65 (2.29, 3.07) | NA |
| Ledipasvir/Sofosbuvir | 90/400 once daily | 25 once daily† | 42 | 1.03 (0.94, 1.14) | 1.32 (1.25, 1.40) | NA |
| Sofosbuvir/velpatasvir/voxilaprevir | 400/100/100 + 100 voxilaprevir‡ once daily | 25 once daily† | 30 | 1.32 (1.17, 1.48) | 1.52 (1.43,1.61) | NA |
| Coadministered Drug | Dose/Schedule | Mean Ratio of Coadministered Drug Pharmacokinetic Parameters With/Without RPV (90% CI); No Effect = 1.00 |
||||
|---|---|---|---|---|---|---|
| Coadministered Drug (mg) | RPV (mg) | N | Cmax | AUC | Cmin | |
| CI=Confidence Interval; N=maximum number of participants with data; NA=Not Available | ||||||
|
||||||
| Acetaminophen | 500 single dose | 150 once daily* | 16 | 0.97 (0.86, 1.10) | 0.92 (0.85, 0.99) | NA |
| Atorvastatin | 40 once daily | 150 once daily* | 16 | 1.35 (1.08, 1.68) | 1.04 (0.97, 1.12) | 0.85 (0.69, 1.03) |
| 2-hydroxy-atorvastatin | 1.58 (1.33, 1.87) | 1.39 (1.29, 1.50) | 1.32 (1.10, 1.58) |
|||
| 4-hydroxy-atorvastatin | 1.28 (1.15, 1.43) | 1.23 (1.13, 1.33) | NA | |||
| Chlorzoxazone | 500 single dose taken 2 hours after RPV | 150 once daily* | 16 | 0.98 (0.85, 1.13) | 1.03 (0.95, 1.13) | NA |
| Digoxin | 0.5 single dose | 25 once daily† | 22 | 1.06 (0.97, 1.17) | 0.98 (0.93, 1.04)‡ | NA |
| Ethinylestradiol | 0.035 once daily | 25 once daily† | 17 | 1.17 (1.06, 1.30) | 1.14 (1.10, 1.19) | 1.09 (1.03, 1.16) |
| Norethindrone | 1 mg once daily | 0.94 (0.83, 1.06) | 0.89 (0.84, 0.94) | 0.99 (0.90, 1.08) |
||
| Ketoconazole | 400 once daily | 150 once daily* | 14 | 0.85 (0.80, 0.90) | 0.76 (0.70, 0.82) | 0.34 (0.25, 0.46) |
| Ledipasvir | 90 once daily | 25 once daily§ | 41 | 1.01 (0.97, 1.05) | 1.02 (0.97, 1.06) | 1.02 (0.98, 1.07) |
| Sofosbuvir | 400 once daily | 25 once daily§ | 41 | 0.96 (0.89, 1.04) | 1.05 (1.01, 1.09) | NA |
| GS-331007¶ | 1.08 (1.05, 1.11) | 1.08 (1.06, 1.10) | 1.10 (1.07, 1.12) |
|||
| R(-) methadone | 60–100 once daily, individualized dose | 25 once daily† | 13 | 0.86 (0.78, 0.95) | 0.84 (0.74, 0.95) | 0.78 (0.67, 0.91) |
| S(+) methadone | 0.87 (0.78, 0.97) | 0.84 (0.74, 0.96) | 0.79 (0.67, 0.92) |
|||
| Metformin | 850 single dose | 25 once daily† | 20 | 1.02 (0.95, 1.10) | 0.97 (0.90,1.06)# | NA |
| Rifampin | 600 once daily | 150 once daily* | 16 | 1.02 (0.93, 1.12) | 0.99 (0.92, 1.07) | NA |
| 25-desacetylrifampin | 1.00 (0.87, 1.15) | 0.91 (0.77, 1.07) | NA | |||
| Simeprevir | 150 once daily | 25 once daily† | 21 | 1.10 (0.97, 1.26) | 1.06 (0.94, 1.19) | 0.96 (0.83, 1.11) |
| Sildenafil | 50 single dose | 75 once daily* | 16 | 0.93 (0.80, 1.08) | 0.97 (0.87, 1.08) | NA |
| N-desmethyl-sildenafil | 0.90 (0.80, 1.02) | 0.92 (0.85, 0.99)‡ | NA | |||
| Sofosbuvir GS-331007¶ | 400 once daily | 25 once dailyÞ | 24 | 1.09 (0.95, 1.25) 0.96 (0.90, 1.01) | 1.16 (1.10, 1.24) 1.04 (1.00, 1.07) |
NA 1.12 (1.07, 1.17) |
| Velpatasvir | 100 once daily | 25 once dailyÞ | 24 | 0.96 (0.85, 1.10) | 0.99 (0.88, 1.11) | 1.02 (0.91, 1.15) |
| Sofosbuvir | 400 once daily | 25 once daily§ | 30 | 0.95 (0.86, 1.05) | 1.01 (0.97, 1.06) | NA |
| GS-331007¶ | 1.02 (0.98, 1.06) | 1.04 (1.01, 1.06) | NA | |||
| Velpatasvir | 100 once daily | 25 once daily§ | 30 | 1.05 (0.96, 1.16) | 1.01 (0.94, 1.07) | 1.01 (0.95, 1.09) |
| Voxilaprevir | 100 + 100 once daily | 25 once daily§ | 30 | 0.96 (0.84, 1.11) | 0.94 (0.84, 1.05) | 1.02 (0.92, 1.12) |
| Coadministered Drug | Dose of Coadministered Drug (mg) | TAF (mg) | N | Mean Ratio of Coadministered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00 |
||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||
| CI=Confidence Interval; N=maximum number of participants with data; NA=Not Available | ||||||
| Midazolam* | 2.5 single dose, orally | 25 once daily†
| 18 | 1.02 (0.92, 1.13) | 1.13 (1.04, 1.23) | NA |
| 1 single dose, IV | 0.99 (0.89, 1.11) | 1.08 (1.04, 1.13) | NA | |||
| Ledipasvir‡ | 90/400 once daily | 25 once daily‡
| 41 | 1.01 (0.97, 1.05) | 1.02 (0.97, 1.06) | 1.02 (0.98,1.07) |
| Sofosbuvir‡ | 0.96 (0.89, 1.04) | 1.05 (1.01, 1.09) | NA | |||
| GS-331007‡,§ | 1.08 (1.05, 1.11) | 1.08 (1.06, 1.10) | 1.10 (1.07, 1.12) | |||
| Norelgestromin | norgestimate 0.180/0.215/0.250 once daily/ethinyl estradiol 0.025 once daily | 25 once daily¶ | 29 | 1.17 (1.07,1.26) | 1.12 (1.07, 1.17) | 1.16 (1.08,1.24) |
| Norgestrel | 1.10 (1.02, 1.18) | 1.09 (1.01, 1.18) | 1.11 (1.03,1.20) |
|||
| Ethinyl estradiol | 1.22 (1.15, 1.29) | 1.11 (1.07, 1.16) | 1.02 (0.93,1.12) |
|||
| Sofosbuvir | 400 once daily | 25 once daily‡ | 30 | 0.95 (0.86, 1.05) | 1.01 (0.97, 1.06) | NA |
| GS-331007§ | 1.02 (0.98, 1.06) | 1.04 (1.01, 1.06) | NA | |||
| Velpatasvir | 100 once daily | 1.05 (0.96, 1.16) | 1.01 (0.94, 1.07) | 1.01 (0.95,1.09) |
||
| Voxilaprevir | 100 + 100 once daily | 0.96 (0.84, 1.11) | 0.94 (0.84, 1.05) | 1.02 (0.92,1.12) |
||
12.4 Microbiology
Mechanism of Action
Emtricitabine: FTC, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase (RT) by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Emtricitabine 5′-triphosphate is a weak inhibitor of mammalian DNA polymerases α, β, Ɛ, and mitochondrial DNA polymerase γ.
Rilpivirine: RPV is a diarylpyrimidine non-nucleoside reverse transcriptase inhibitor of HIV-1 and inhibits HIV-1 replication by non-competitive inhibition of HIV-1 RT. RPV does not inhibit the human cellular DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Tenofovir Alafenamide: TAF is a phosphonamidate prodrug of tenofovir (2'-deoxyadenosine monophosphate analog). Plasma exposure to TAF allows for permeation into cells and then TAF is intracellularly converted to tenofovir through hydrolysis by cathepsin A. Tenofovir is subsequently phosphorylated by cellular kinases to the active metabolite tenofovir diphosphate. Tenofovir diphosphate inhibits HIV-1 replication through incorporation into viral DNA by the HIV reverse transcriptase, which results in DNA chain termination.
Tenofovir has activity against human immunodeficiency virus (HIV-1). Cell culture studies have shown that both tenofovir and FTC can be fully phosphorylated when combined in cells. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases that include mitochondrial DNA polymerase γ and there is no evidence of toxicity to mitochondria cell culture.
Antiviral Activity in Cell Culture
Emtricitabine, Rilpivirine, and Tenofovir Alafenamide: The combinations of FTC, RPV, and TAF were not antagonistic with each other in cell culture combination antiviral activity assays. In addition, FTC, RPV, and TAF were not antagonistic with a panel of representatives from the major classes of approved anti-HIV agents (NNRTIs, NRTIs, INSTIs, and PIs).
Emtricitabine: The antiviral activity of FTC against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, the MAGI-CCR5 cell line, and primary peripheral blood mononuclear cells (PBMCs). The EC50 values for FTC were in the range of 1.3–640 nM. FTC displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC50 values ranged from 7–75 nM) and showed strain-specific activity against HIV-2 (EC50 values ranged from 7–1500 nM).
Rilpivirine: RPV exhibited activity against laboratory strains of wild-type HIV-1 in an acutely infected T-cell line with a median EC50 value for HIV-1IIIB of 0.73 nM. RPV demonstrated limited activity in cell culture against HIV-2 with a median EC50 value of 5220 nM (range 2510–10,830 nM). RPV demonstrated antiviral activity against a broad panel of HIV-1 group M (subtype A, B, C, D, F, G, H) primary isolates with EC50 values ranging from 0.07–1.01 nM and was less active against group O primary isolates with EC50 values ranging from 2.88–8.45 nM.
Tenofovir Alafenamide: The antiviral activity of TAF against laboratory and clinical isolates of HIV-1 subtype B was assessed in lymphoblastoid cell lines, PBMCs, primary monocyte/macrophage cells and CD4-T lymphocytes. The EC50 values for TAF ranged from 2.0–14.7 nM.
TAF displayed antiviral activity in cell culture against all HIV-1 groups (M, N, O), including sub-types A, B, C, D, E, F, and G (EC50 values ranged from 0.10–12.0 nM) and strain specific activity against HIV-2 (EC50 values ranged from 0.91–2.63 nM).
Resistance
In Cell Culture
Emtricitabine: HIV-1 isolates with reduced susceptibility to FTC were selected in cell culture. Reduced susceptibility to FTC was associated with M184V or I substitutions in HIV-1 RT.
Rilpivirine: RPV-resistant strains were selected in cell culture starting from wild-type HIV-1 of different origins and subtypes as well as NNRTI-resistant HIV-1. The frequently observed amino acid substitutions that emerged and conferred decreased phenotypic susceptibility to RPV included: L100I, K101E, V106I and A, V108I, E138K and G, Q, R, V179F and I, Y181C and I, V189I, G190E, H221Y, F227C, and M230I and L.
In Clinical Trials
In Participants With HIV-1 and No Antiretroviral Treatment History
Emtricitabine and Tenofovir Alafenamide: The resistance profile of ODEFSEY for the treatment of HIV-1 is based on studies of FTC+TAF with EVG+COBI in the treatment of HIV-1. In a pooled analysis of antiretroviral-naïve participants, genotyping was performed on plasma HIV-1 isolates from all participants with HIV-1 RNA greater than 400 copies per mL at confirmed virologic failure, at Week 48, or at time of early study drug discontinuation. Genotypic resistance developed in 7 of 14 evaluable participants. The resistance–associated substitutions that emerged were M184V/I (N=7) and K65R (N=1). Three participants had virus with emergent R, H, or E at the polymorphic Q207 residue in reverse transcriptase.
Rilpivirine: In the Week 96 pooled resistance analysis for adult participants receiving RPV or efavirenz in combination with FTC/TDF, the emergence of resistance was greater among participants' viruses in the RPV+FTC/TDF arm compared to the efavirenz+FTC/TDF arm and was dependent on baseline viral load. In the Week 96 resistance analysis, 14% (77/550) of the participants in the RPV+FTC/TDF arm and 8% (43/546) of the participants in the efavirenz+FTC/TDF arm qualified for resistance analysis; 61% (47/77) of the participants who qualified for resistance analysis (resistance-analysis participants) in the RPV+FTC/TDF arm had virus with genotypic and/or phenotypic resistance to RPV compared to 42% (18/43) of the resistance-analysis participants in the efavirenz+FTC/TDF arm who had genotypic and/or phenotypic resistance to efavirenz. Moreover, genotypic and/or phenotypic resistance to emtricitabine or tenofovir emerged in viruses from 57% (44/77) of the resistance-analysis participants in the RPV arm compared to 26% (11/43) in the efavirenz arm.
Emerging NNRTI substitutions in the RPV resistance analysis of participants' viruses included V90I, K101E/P/T, E138K/A/Q/G, V179I/L, Y181C/I, V189I, H221Y, F227C/L, and M230L, which were associated with an RPV phenotypic fold change range of 2.6–621. The E138K substitution emerged most frequently during RPV treatment, commonly in combination with the M184I substitution. The emtricitabine and lamivudine resistance-associated substitutions M184I or V and NRTI resistance-associated substitutions (A62V, K65R/N, D67N/G, K70E, Y115F, K219E/R) emerged more frequently in the RPV resistance-analysis participants than in efavirenz resistance-analysis participants.
NNRTI- and NRTI-resistance substitutions emerged less frequently in the resistance analysis of viruses from participants with baseline viral loads of less than or equal to 100,000 copies/mL compared to viruses from participants with baseline viral loads of greater than 100,000 copies/mL: 23% (10/44) compared to 77% (34/44) of NNRTI-resistance substitutions and 20% (9/44) compared to 80% (35/44) of NRTI-resistance substitutions. This difference was also observed for the individual emtricitabine/lamivudine and tenofovir resistance substitutions: 22% (9/41) compared to 78% (32/41) for M184I/V and 0% (0/8) compared to 100% (8/8) for K65R/N. Additionally, NNRTI and/or NRTI-resistance substitutions emerged less frequently in the resistance analysis of the viruses from participants with baseline CD4+ cell counts greater than or equal to 200 cells/mm3 compared to the viruses from participants with baseline CD4+ cell counts less than 200 cells/mm3: 32% (14/44) compared to 68% (30/44) of NNRTI-resistance substitutions and 27% (12/44) compared to 73% (32/44) of NRTI-resistance substitutions.
In Virologically-Suppressed Participants
Emtricitabine and Tenofovir Alafenamide: One participant was identified with emergent resistance to FTC or TAF (M184M/I) out of 4 virologic failure participants in a clinical study of virologically-suppressed participants who switched from a regimen containing FTC+TDF to FTC+TAF with EVG+COBI (N=799).
Rilpivirine: Through Week 48, 4 participants who switched their protease inhibitor-based regimen to FTC/RPV/TDF (4 of 469 participants, 0.9%) and 1 participant who maintained their regimen (1 of 159 participants, 0.6%) developed genotypic and/or phenotypic resistance to a study drug. All 4 of the participants who had resistance emergence on FTC/RPV/TDF had evidence of FTC resistance and 3 of the participants had evidence of RPV resistance.
ODEFSEY: Through Week 48, in participants who switched to ODEFSEY from FTC/RPV/TDF or EFV/FTC/TDF (Trials 1216 (N=316) and 1160 (N=438), respectively), of seven participants who developed virologic failure, three participants had detectable NNRTI and/or NRTI resistance substitutions at virologic failure that were pre-existing in the baseline sample by proviral DNA sequencing; one of these participants resuppressed while maintaining ODEFSEY.
Cross-Resistance
Emtricitabine: FTC-resistant viruses with the M184V/I substitution were cross-resistant to lamivudine, but retained sensitivity to didanosine, stavudine, tenofovir, and zidovudine.
Viruses harboring substitutions conferring reduced susceptibility to stavudine and zidovudine—thymidine analog substitutions (M41L, D67N, K70R, L210W, T215Y/F, K219Q/E), or didanosine (L74V) remained sensitive to FTC. HIV-1 containing the K103N substitution or other substitutions associated with resistance to NNRTIs was susceptible to FTC.
Rilpivirine: Considering all of the available cell culture and clinical data, any of the following amino acid substitutions, when present at baseline, are likely to decrease the antiviral activity of RPV: K101E, K101P, E138A, E138G, E138K, E138R, E138Q, V179L, Y181C, Y181I, Y181V, Y188L, H221Y, F227C, M230I, M230L, and the combination of L100I+K103N.
Cross-resistance in site-directed mutant virus has been observed among NNRTIs. The single NNRTI substitutions K101P, Y181I, and Y181V conferred 52 times, 15 times, and 12 times decreased susceptibility to RPV, respectively. The combination of E138K and M184I showed 6.7 times reduced susceptibility to RPV compared to 2.8 times for E138K alone. The K103N substitution did not show reduced susceptibility to RPV by itself. However, the combination of L100I and K103N resulted in a 7 times reduced susceptibility to RPV. In another study, the Y188L substitution resulted in a reduced susceptibility to RPV of 9 times for clinical isolates and 6 times for site-directed mutants. Combinations of 2 or 3 NNRTI resistance-associated substitutions gave decreased susceptibility to RPV (fold change range of 3.7–554) in 38% and 66% of mutants, respectively.
Cross-resistance to efavirenz, etravirine, and/or nevirapine is likely after virologic failure and development of RPV resistance.
Tenofovir Alafenamide: Tenofovir resistance substitutions K65R and K70E result in reduced susceptibility to abacavir, didanosine, emtricitabine, lamivudine, and tenofovir.
HIV-1 with multiple thymidine analog substitutions (M41L, D67N, K70R, L210W, T215F/Y, K219Q/E/N/R), or multinucleoside resistant HIV-1 with a T69S double insertion mutation or with a Q151M substitution complex including K65R showed reduced susceptibility to TAF in cell culture.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Emtricitabine: In long-term carcinogenicity studies of FTC, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg per kg per day (23 times the human systemic exposure at the recommended dose of 200 mg per day in ODEFSEY) or in rats at doses up to 600 mg per kg per day (28 times the human systemic exposure at the recommended dose in ODEFSEY).
FTC was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or mouse micronucleus assays.
FTC did not affect fertility in male rats at approximately 140 times or in male and female mice at approximately 60 times higher exposures (AUC) than in humans given the recommended 200 mg daily dose in ODEFSEY. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the recommended 200 mg daily dose in ODEFSEY.
Rilpivirine: RPV was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks. Daily doses of 20, 60, and 160 mg per kg per day were administered to mice and doses of 40, 200, 500, and 1500 mg per kg per day were administered to rats. In rats, there were no drug-related neoplasms. In mice, RPV was positive for hepatocellular neoplasms in both males and females. The observed hepatocellular findings in mice may be rodent-specific. At the lowest tested doses in the carcinogenicity studies, the systemic exposures (based on AUC) to RPV were 21 times (mice) and 3 times (rats) relative to those observed in humans at the recommended dose (25 mg once daily) in ODEFSEY.
RPV has tested negative in the absence and presence of a metabolic activation system, in the in vitro Ames reverse mutation assay and in vitro clastogenicity mouse lymphoma assay. RPV did not induce chromosomal damage in the in vivo micronucleus test in mice.
In a study conducted in rats, there were no effects on mating or fertility with RPV up to 400 mg per kg per day, a dose of RPV that showed maternal toxicity. This dose is associated with an exposure that is approximately 40 times higher than the exposure in humans at the recommended dose of 25 mg once daily in ODEFSEY.
Tenofovir Alafenamide: Since TAF is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice was observed after TAF administration compared to TDF administration, carcinogenicity studies were conducted only with TDF. Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the recommended dose of TDF (300 mg) for HIV-1 infection. The tenofovir exposure in these studies was approximately 167 times (mice) and 55 times (rat) those observed in humans after administration of the daily recommended dose of ODEFSEY. At the high dose in female mice, liver adenomas were increased at tenofovir exposures approximately 10 times (300 mg TDF) and 167 times (ODEFSEY) the exposure observed in humans. In rats, the study was negative for carcinogenic findings.
TAF was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
There were no effects on fertility, mating performance or early embryonic development when TAF was administered to male rats at a dose equivalent to 62 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 14 days prior to mating through Day 7 of gestation.
13.2 Animal Toxicology and/or Pharmacology
Minimal to slight infiltration of mononuclear cells in the posterior uvea was observed in dogs with similar severity after three- and nine-month administration of TAF; reversibility was seen after a three-month recovery period. No eye toxicity was observed in the dog at systemic exposures of 5 (TAF) and 15 (tenofovir) times the exposure seen in humans at the recommended daily TAF dose in ODEFSEY.
14. Clinical Studies
14.1 Clinical Trial Results in HIV-1 Virologically-Suppressed Subjects Who Switched to ODEFSEY
In Trial 1216, the efficacy and safety of switching from emtricitabine/rilpivirine/tenofovir disoproxil fumarate (FTC/RPV/TDF) to ODEFSEY were evaluated in a randomized, double-blind study of virologically-suppressed adults with HIV-1. Participants were suppressed (HIV-1 RNA <50 copies/mL) on their baseline regimen of FTC/RPV/TDF for at least 6 months and had no documented resistance mutations to FTC, TAF, or RPV prior to study entry. Participants were randomized in a 1:1 ratio to either switch to ODEFSEY (N=316) once daily or stay on FTC/RPV/TDF (N=314) once daily. Participants had a mean age of 45 years (range: 23–72), 90% were male, 75% were White, and 19% were Black. The mean baseline CD4+ cell count was 709 cells/mm3 (range: 104–2527).
In Trial 1160, the efficacy and safety of switching from efavirenz/emtricitabine/tenofovir disoproxil fumarate (EFV/FTC/TDF) to ODEFSEY were evaluated in a randomized, double-blind study of virologically-suppressed adults with HIV-1. Participants were stably suppressed (HIV-1 RNA <50 copies/mL) on their baseline regimen of EFV/FTC/TDF for at least 6 months and had no documented resistance mutations to FTC, TAF, or RPV prior to study entry. Participants were randomized in a 1:1 ratio to either switch to ODEFSEY (N=438) once daily or stay on EFV/FTC/TDF (N=437) once daily. Participants had a mean age of 48 years (range: 19–76), 87% were male, 67% were White, and 27% were Black. The mean baseline CD4+ cell count was 700 cells/mm3 (range: 140–1862).
Treatment outcomes of Trials 1216 and 1160 are presented in Table 13.
| Study 1216 | Study 1160 | |||
|---|---|---|---|---|
| ODEFSEY (N=316) | FTC/RPV/TDF (N=313)† | ODEFSEY (N=438) | EFV/FTC/TDF (N=437) |
|
|
||||
| HIV-1 RNA <50 copies/mL | 94% | 94% | 90% | 92% |
| HIV-1 RNA ≥50 copies/mL‡ | 1% | 0% | 1% | 1% |
| No Virologic Data at Week 48 Window | 6% | 6% | 9% | 7% |
| Discontinued Study Drug Due to AE or Death and Last Available HIV-1 RNA <50 copies/mL | 2% | 1% | 3% | 1% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA <50 copies/mL§ | 4% | 4% | 5% | 5% |
| Missing Data During Window but on Study Drug | <1% | 1% | 1% | 1% |
14.2 Clinical Trial Results for Adult Participants with no Antiretroviral Treatment History and Adults with Renal Impairment for Components of ODEFSEY
The efficacy of RPV, FTC, and TAF in the treatment of HIV-1 infection in adults as initial therapy in those with no antiretroviral treatment history [see Indications and Usage (1)] was established in trials of:
- RPV+FTC/TDF in adults with HIV-1 as initial therapy in those with no antiretroviral treatment history (n=550). The virologic response rate (i.e., HIV-1 RNA less than 50 copies per mL) was 77% at Week 96. The virologic response rate at 96 weeks was 83% in participants with baseline HIV-1 RNA less than or equal to 100,000 copies per mL and 71% in participants with baseline HIV-1 RNA greater than 100,000 copies per mL. Further, the virologic response rate at 96 weeks among participants with baseline CD4+ cell counts less than 200 and greater than or equal to 200 cells/mm3 were 68% and 82%, respectively.
- FTC+TAF with EVG+COBI in adults with HIV-1 as initial therapy in those with no antiretroviral treatment history (n=866). The virologic response rate (i.e., HIV-1 RNA less than 50 copies per mL) was 92% at Week 48.
In the clinical trial of 248 adults with HIV-1 and estimated creatinine clearance greater than 30 mL per minute but less than 70 mL per minute, 95% (235/248) of the combined populations of treatment-naïve (N=6) begun on FTC+TAF with EVG+COBI and those previously virologically-suppressed on other regimens (N=242) and switched to FTC+TAF with EVG +COBI had HIV-1 RNA levels less than 50 copies per mL at Week 24.
14.3 Clinical Trial Results for Pediatric Participants Aged 6 to Less than 18 Years Old for Components of ODEFSEY
The efficacy of RPV, FTC, and TAF in the treatment of HIV-1 infection in pediatric patients aged 6 to less than 18 years old and greater than 25 kg as initial therapy in those with no antiretroviral treatment history and to replace a stable antiretroviral regimen in those who are virologically-suppressed [see Indications and Usage (1)] was established in trials of pediatric participants with HIV-1 aged 6 to less than 18 years with:
- RPV in combination with other antiretroviral agents in 36 treatment-naïve adolescents with HIV-1 weighing at least 32 kg. The majority of participants (24/36) received RPV in combination with FTC and TDF. Of these 24 participants, 20 had a baseline HIV-1 RNA less than or equal to 100,000 copies per mL. The virologic response rate in these 20 participants (i.e., HIV-1 RNA less than 50 copies per mL) was 80% (16/20) at 48 weeks.
- RPV in combination with other antiretroviral agents (two NRTIs) in 18 treatment-naïve pediatric participants with HIV-1 weighing at least 17 kg (median [range]: 25 kg [17–51 kg]). The virologic response rate (i.e., HIV-1 RNA less than 50 copies per mL) was 72% (13/18) at 48 weeks.
- RPV in combination with other antiretrovirals in 26 virologically-suppressed pediatric participants with HIV-1 less than 12 years old weighing at least 16 kg (N=26) at 48-week.
- FTC+TAF with EVG+COBI in 50 treatment-naïve adolescents with HIV-1 aged 12 to less than 18 years weighing at least 35 kg. The virologic response rate (i.e., HIV-1 RNA less than 50 copies per mL) was 92% (46/50) at 48 weeks.
- FTC+TAF with EVG+COBI in 52 virologically-suppressed pediatric participants with HIV-1 aged 6 to less than 12 years weighing at least 25 kg. The virologic response rate (i.e., HIV-1 RNA less than 50 copies per mL) was 98% (51/52) at 48 weeks.
16. How is Odefsey supplied
ODEFSEY tablets are gray, capsule-shaped, and film coated with "GSI" debossed on one side and "255" on the other side. Each bottle contains 30 tablets (NDC 61958-2101-1), a silica gel desiccant, and a polyester coil, and is closed with a child-resistant closure.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Post-treatment Acute Exacerbation of Hepatitis B in Patients with HBV Coinfection
Severe acute exacerbations of hepatitis B have been reported in patients with HBV and HIV-1 who have discontinued products containing FTC and/or TDF, and may likewise occur with discontinuation of ODEFSEY [see Warnings and Precautions (5.1)]. Advise the patient to not discontinue ODEFSEY without first informing their healthcare provider.
Severe Skin Reactions and Hypersensitivity
Inform patients that skin reactions ranging from mild to severe, including Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), have been reported with RPV-containing products. Instruct patients to immediately stop taking ODEFSEY and seek medical attention if they develop a rash associated with any of the following symptoms: fever, blisters, mucosal involvement, eye inflammation (conjunctivitis), severe allergic reaction causing swelling of the face, eyes, lips, mouth, tongue or throat which may lead to difficulty swallowing or breathing, and any signs and symptoms of liver problems, as they may be a sign of a more serious reaction. Patients should understand that if severe rash occurs, they will be closely monitored, laboratory tests will be performed and appropriate therapy will be initiated [see Warnings and Precautions (5.2)].
Hepatotoxicity
Inform patients that hepatotoxicity has been reported with RPV, therefore, it is important to inform the healthcare professional if patients have underlying hepatitis B or C or elevations in liver-associated tests prior to treatment [see Dosage and Administration (2.1) and Warnings and Precautions (5.3)].
Depressive Disorders
Inform patients that depressive disorders (depressed mood, depression, dysphoria, major depression, mood altered, negative thoughts, suicide attempt, suicidal ideation) have been reported with RPV. Inform patients to seek immediate medical evaluation if they experience depressive symptoms [see Warnings and Precautions (5.4)].
New Onset or Worsening Renal Impairment
Advise patients to avoid taking ODEFSEY with concurrent or recent use of nephrotoxic agents. Postmarketing cases of renal impairment, including acute renal failure, have been reported [see Warnings and Precautions (5.5)].
Drug Interactions
ODEFSEY may interact with many drugs and is not recommended to be coadministered with numerous drugs. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's wort [see Contraindications (4), Warnings and Precautions (5.6) and Drug Interactions (7)].
Lactic Acidosis and Severe Hepatomegaly
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with use of drugs similar to ODEFSEY. Advise patients to stop taking ODEFSEY if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.7)].
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.8)].
Missed Dosage
Inform patients that it is important to take ODEFSEY on a regular dosing schedule with a meal and to avoid missing doses, as it can result in development of resistance [see Dosage and Administration (2.2)].
Pregnancy Registry
Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes in those exposed to ODEFSEY during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct patients with HIV-1 that the potential risks of breastfeeding include: (1) HIV-1 transmission to infants without HIV-1, (2) developing viral resistance in infants with HIV-1, and (3) adverse reactions in a breastfed infant similar to those seen in adults [see Use in Specific Populations (8.2)].
ODEFSEY is a trademark of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners.
| Patient Information ODEFSEY® (oh-DEF-see) (emtricitabine, rilpivirine and tenofovir alafenamide) tablets |
|||
|---|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Revised: 02/2025 | ||
| Important: Ask your healthcare provider or pharmacist about medicines that should not be taken with ODEFSEY. For more information, see "What should I tell my healthcare provider before taking ODEFSEY?" | |||
| What is the most important information I should know about ODEFSEY? ODEFSEY can cause serious side effects, including:
|
|||
| What is ODEFSEY?
ODEFSEY is a prescription medicine that is used to treat human immunodeficiency virus-1 (HIV-1) infection in adults and children who weigh at least 55 pounds (25 kg):
ODEFSEY contains the prescription medicines emtricitabine, rilpivirine and tenofovir alafenamide. It is not known if ODEFSEY is safe and effective in children who weigh less than 55 pounds (25 kg). |
|||
| Who should not take ODEFSEY? Do not take ODEFSEY if you also take a medicine that contains: |
|||
|
|
||
| What should I tell my healthcare provider before taking ODEFSEY? Before taking ODEFSEY, tell your healthcare provider about all of your medical conditions, including if you:
Some medicines may interact with ODEFSEY. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
|
|||
How should I take ODEFSEY?
|
|||
| What are the possible side effects of ODEFSEY? ODEFSEY may cause serious side effects, including:
|
|||
|
|
||
These are not all of the possible side effects of ODEFSEY. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store ODEFSEY?
|
|||
| General information about the safe and effective use of ODEFSEY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ODEFSEY for a condition for which it was not prescribed. Do not give ODEFSEY to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ODEFSEY that is written for health professionals. |
|||
| What are the ingredients in ODEFSEY?
Active ingredients: emtricitabine, rilpivirine, and tenofovir alafenamide. Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, and povidone. The tablet film coating contains iron oxide black, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Manufactured and distributed by: Gilead Sciences, Inc. Foster City, CA 94404 ODEFSEY is a trademark of Gilead Sciences, Inc., or its related companies. © 2025 Gilead Sciences, Inc. All rights reserved. 208351-GS-007 For more information, call 1-800-445-3235 or go to www.ODEFSEY.com. |
|||
PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
NDC 61958- 2101-1
30 tablets
Odefsey ®
(emtricitabine, rilpivirine, and
tenofovir alafenamide) Tablets
200 mg/25 mg/25 mg
Note to pharmacist:
Do not cover ALERT box with pharmacy label.
ALERT: Find out about medicines that
should NOT be taken with Odefsey ®
| ODEFSEY
emtricitabine, rilpivirine hydrochloride, and tenofovir alafenamide tablet |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |
Frequently asked questions
More about Odefsey (emtricitabine / rilpivirine / tenofovir alafenamide)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (10)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: antiviral combinations

