Nexletol: Package Insert / Prescribing Info
Package insert / product label
Generic name: bempedoic acid
Dosage form: tablet, film coated
Drug class: Miscellaneous antihyperlipidemic agents
Medically reviewed by Drugs.com. Last updated on Apr 15, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
NEXLETOL (bempedoic acid) tablets, for oral use
Initial U.S. Approval: 2020
Recent Major Changes
Indications and Usage for Nexletol
NEXLETOL, an adenosine triphosphate-citrate lyase (ACL) inhibitor, is indicated:
- To reduce the risk of myocardial infarction and coronary revascularization in adults who are unable to take recommended statin therapy (including those not taking a statin) with:
- established cardiovascular disease (CVD), or
- a high risk for a CVD event but without established CVD. (1)
- As an adjunct to diet, in combination with other low-density lipoprotein cholesterol (LDL-C) lowering therapies, or alone when concomitant LDL-C lowering therapy is not possible, to reduce LDL-C in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH). (1)
Nexletol Dosage and Administration
Administer 180 mg orally once daily with or without food. (2.1)
Dosage Forms and Strengths
Tablets: 180 mg (3)
Contraindications
History of a serious hypersensitivity reaction to bempedoic acid or any of the excipients in NEXLETOL. (4)
Warnings and Precautions
Hyperuricemia: Elevations in serum uric acid have occurred. Assess uric acid levels periodically as clinically indicated. Monitor for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate. (5.1)
Tendon Rupture: Tendon rupture has occurred. Discontinue NEXLETOL at the first sign of tendon rupture. Avoid NEXLETOL in patients who have a history of tendon disorders or tendon rupture. (5.2)
Adverse Reactions/Side Effects
Common adverse reactions in the (6.1):
- Primary hyperlipidemia trials (incidence ≥ 2% and more frequent than placebo) were upper respiratory tract infection, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes.
- Cardiovascular outcomes trial (incidence ≥ 2% and 0.5% greater than placebo) were hyperuricemia, renal impairment, anemia, elevated liver enzymes, muscle spasms, gout, and cholelithiasis.
To report SUSPECTED ADVERSE REACTIONS, contact Esperion at 833-377-7633 (833 ESPRMED) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2024
Full Prescribing Information
1. Indications and Usage for Nexletol
NEXLETOL is indicated:
-
To reduce the risk of myocardial infarction and coronary revascularization in adults who are unable to take recommended statin therapy (including those not taking a statin) with:
- established cardiovascular disease (CVD), or
- a high risk for a CVD event but without established CVD.
- As an adjunct to diet, in combination with other low-density lipoprotein cholesterol (LDL-C) lowering therapies, or alone when concomitant LDL-C lowering therapy is not possible, to reduce LDL-C in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH).
3. Dosage Forms and Strengths
NEXLETOL is available as:
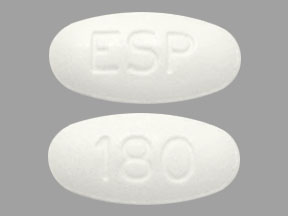
- Tablets: 180 mg, white to off-white, oval shaped, debossed with "180" on one side and "ESP" on the other side.
4. Contraindications
NEXLETOL is contraindicated in patients with a prior serious hypersensitivity reaction to bempedoic acid or any of the excipients in NEXLETOL. Serious hypersensitivity reactions, such as angioedema, have occurred [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Hyperuricemia
NEXLETOL inhibits renal tubular OAT2 and may increase blood uric acid levels [see Clinical Pharmacology (12.3)]. In the primary hyperlipidemia trials [see Clinical Studies (14.2)], 26% of NEXLETOL-treated patients with normal baseline uric acid values (versus 9.5% placebo) experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction (versus 1.1% placebo). Increases in uric acid levels usually occurred within the first 4 weeks of treatment initiation, persisted throughout treatment, and returned to baseline following discontinuation of treatment. After 12 weeks of treatment, the mean placebo-adjusted increase in uric acid compared to baseline was 0.8 mg/dL for patients treated with NEXLETOL. In the cardiovascular outcomes trial [see Clinical Studies (14.1)], 16.4% of NEXLETOL-treated patients experienced clinically significant hyperuricemia reported as an adverse reaction (versus 8.2% placebo).
Elevated blood uric acid may lead to the development of gout. In the primary hyperlipidemia trials, gout was reported in 1.5% of patients treated with NEXLETOL and 0.4% of patients treated with placebo. In the cardiovascular outcomes trial, gout was reported in 3.2% of patients treated with NEXLETOL and 2.2% treated with placebo.
Advise patients to contact their healthcare provider if symptoms of hyperuricemia occur. Assess serum uric acid when clinically indicated. Monitor patients for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate.
5.2 Tendon Rupture
NEXLETOL is associated with an increased risk of tendon rupture or injury. In the primary hyperlipidemia trials [see Clinical Studies (14.2)], tendon rupture occurred in 0.5% of patients treated with NEXLETOL versus 0% of placebo-treated patients and involved the rotator cuff (the shoulder), biceps tendon, or Achilles tendon. Tendon rupture occurred within weeks to months of starting NEXLETOL. In the cardiovascular outcomes trial [see Clinical Studies (14.1)], tendon rupture events occurred in 1.2% of NEXLETOL-treated patients versus 0.9% of placebo-treated patients. Tendon rupture may occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders.
Discontinue NEXLETOL immediately if the patient experiences rupture of a tendon. Consider discontinuing NEXLETOL if the patient experiences joint pain, swelling, or inflammation. Advise patients to rest at the first sign of tendinitis or tendon rupture and to contact their healthcare provider if tendinitis or tendon rupture symptoms occur. Consider alternative therapy in patients with a history of tendon disorders or tendon rupture.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hyperuricemia [see Warnings and Precautions (5.1)]
- Tendon Rupture [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data in Table 1 reflect exposure to NEXLETOL in two placebo-controlled primary hyperlipidemia trials that included 2,009 patients treated with NEXLETOL for 52 weeks (median treatment duration of 52 weeks) [see Clinical Studies (14.2)]. The mean age for NEXLETOL-treated patients was 65 years, 29% were female, 95% were White, 3% were Black or African American, 1% were Asian, and 1% were other races; 3% identified as Hispanic or Latino ethnicity. All patients received NEXLETOL 180 mg orally once daily plus maximally tolerated statin therapy alone or in combination with other lipid-lowering therapies. At baseline, 97% of patients had CVD and about 4% had a diagnosis of HeFH. Patients on simvastatin 40 mg/day or higher were excluded from the trials.
In the primary hyperlipidemia trials, adverse reactions led to discontinuation of treatment in 11% of NEXLETOL-treated patients and 8% of placebo-treated patients. The most common reasons for NEXLETOL treatment discontinuation were muscle spasms (0.5% versus 0.3% placebo), diarrhea (0.4% versus 0.1% placebo), and pain in extremity (0.3% versus 0.0% placebo). Adverse reactions reported in at least 2% of NEXLETOL-treated patients and more frequently than in placebo-treated patients are shown in Table 1.
| Adverse Reaction | Placebo*
(N = 999) % | NEXLETOL*
(N = 2,009) % |
|---|---|---|
| Upper respiratory tract infection | 4.0 | 4.5 |
| Muscle spasms | 2.3 | 3.6 |
| Hyperuricemia† | 1.1 | 3.5 |
| Back pain | 2.2 | 3.3 |
| Abdominal pain or discomfort† | 2.2 | 3.1 |
| Bronchitis | 2.5 | 3.0 |
| Pain in extremity | 1.7 | 3.0 |
| Anemia | 1.9 | 2.8 |
| Elevated liver enzymes† | 0.8 | 2.1 |
In the cardiovascular outcomes trial, in which 7,001 patients were exposed to NEXLETOL and 6,964 patients were exposed to placebo for a median of 3.1 years [see Clinical Studies, (14.1)], adverse reactions led to discontinuation of treatment in 11% of NEXLETOL-treated patients and 10% of placebo-treated patients. Adverse reactions reported in at least 2% of NEXLETOL-treated patients and 0.5% greater than than placebo are shown in Table 2.
| Adverse Reaction | Placebo (N=6,964) % | NEXLETOL (N=7,001) % |
|---|---|---|
| Hyperuricemia* | 8 | 16 |
| Renal impairment† | 9 | 11 |
| Anemia | 4 | 5 |
| Elevated liver enzymes* | 3 | 4 |
| Muscle spasms | 3 | 4 |
| Gout | 2 | 3 |
| Cholelithiasis | 1 | 2 |
Other Adverse Reactions
Laboratory Tests
NEXLETOL was associated with persistent changes in multiple laboratory tests that occurred within the first 4 weeks of treatment and returned to baseline following discontinuation of treatment.
Increase in Creatinine and Blood Urea Nitrogen
In the hyperlipidemia trials, there was a mean increase in serum creatinine of 0.05 mg/dL compared to baseline with NEXLETOL at Week 12. Approximately 3.8% of patients treated with NEXLETOL had blood urea nitrogen values that doubled (versus 1.5% placebo), and about 2.2% of patients had creatinine values that increased by 0.5 mg/dL (versus 1.1% placebo). In the cardiovascular outcomes trial, 7.1% of patients had creatinine values that increased by 0.5 mg/dL (versus 5.5% placebo) and 9.5% of patients in the NEXLETOL group had BUN values that increased ≥ 2× baseline (versus 6.2% placebo).
Decrease in Hemoglobin and Leukocytes
In the hyperlipidemia trials, approximately 5.1% of patients treated with NEXLETOL (versus 2.3% placebo) had decreases in hemoglobin levels of 2 or more g/dL and below the lower limit of normal on one or more occasion. Anemia was reported in 2.8% of patients treated with NEXLETOL and 1.9% of patients treated with placebo. Approximately 9.0% of NEXLETOL-treated patients with normal baseline leukocyte count had a decrease to less than the lower limit of normal on one or more occasion (versus 6.7% placebo). Leukocyte decrease was generally asymptomatic and did not require medical intervention. In the hyperlipidemia trials, there was a small imbalance in skin or soft tissue infections, including cellulitis (0.8% versus 0.4%), but there was no imbalance in other infections.
In the cardiovascular outcomes trial, 10.8% of patients (versus 7.4% placebo) had a decrease in hemoglobin of 2 or more g/dL and below the lower limit of normal. Anemia was reported in 4.7% of patients treated with NEXLETOL and 3.9% of patients treated with placebo. There were 9.3% of NEXLETOL-treated patients with a leukocyte count below the lower limit of normal (and normal at baseline) at any point (versus 6.8% placebo).
Increase in Platelet Count
In the hyperlipidemia trials, approximately 10.1% of patients (versus 4.7% placebo) had increases in platelet counts of 100× 109/L or more on one or more occasion. In the cardiovascular outcomes trial, 18.6% of patients in the NEXLETOL-treated group (versus 10.2% placebo) had an increase in platelet count of 100 × 109/L or more. Platelet count increase was asymptomatic and did not result in increased risk for thromboembolic events.
Increase in Liver Enzymes
In the hyperlipidemia trials, increases in hepatic transaminases (AST and/or ALT) were observed with NEXLETOL. In most cases, the elevations were transient and resolved or improved with continued therapy or after discontinuation of therapy. Increases to more than 3× the upper limit of normal (ULN) in AST occurred in 1.4% of patients treated with NEXLETOL versus 0.4% of placebo patients, and increases to more than 5× ULN occurred in 0.4% of NEXLETOL-treated versus 0.2% of placebo-treated patients. Increases in ALT occurred with similar incidence between NEXLETOL- and placebo-treated patients. Elevations in transaminases were generally asymptomatic and not associated with elevations ≥ 2× ULN in bilirubin or with cholestasis.
In the cardiovascular outcomes trial, the incidence of repeated and confirmed ALT and/or AST >3× ULN was 1.6% in the NEXLETOL-treated group (versus 1.0% placebo). A higher percentage of patients in the NEXLETOL-treated group had hepatic enzyme elevations versus placebo (4.5% versus 3.0%, respectively).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of NEXLETOL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity reactions including: angioedema, wheezing, rash, and urticaria.
7. Drug Interactions
Table 3 includes a list of drugs with clinically important drug interactions when administered concomitantly with NEXLETOL and instructions for preventing or managing them.
| Simvastatin | |
| Clinical Impact: | Concomitant use of NEXLETOL with simvastatin causes an increase in simvastatin concentration and may increase the risk of simvastatin-related myopathy [see Clinical Pharmacology (12.3)]. |
| Intervention: | Avoid concomitant use of NEXLETOL with simvastatin greater than 20 mg. |
| Pravastatin | |
| Clinical Impact: | Concomitant use of NEXLETOL with pravastatin causes an increase in pravastatin concentration and may increase the risk of pravastatin-related myopathy [see Clinical Pharmacology (12.3)]. |
| Intervention: | Avoid concomitant use of NEXLETOL with pravastatin greater than 40 mg. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Discontinue NEXLETOL when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus.
There are insufficient data on NEXLETOL use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, bempedoic acid was not teratogenic in rats and rabbits when administered at doses resulting in exposures up to 11 and 12 times, respectively, the human exposures at the maximum clinical dose, based on AUC (see Data). NEXLETOL decreases cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol; therefore, NEXLETOL may cause fetal harm when administered to pregnant women based on the mechanism of action [see Clinical Pharmacology (12.1)]. In addition, treatment of hyperlipidemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Report pregnancies to the Esperion Therapeutics, Inc. Adverse Event reporting line at 1-833-377-7633.
Data
Animal Data
Bempedoic acid was not teratogenic when given orally at doses of 60 and 80 mg/kg/day, resulting in 11 and 12 times the systemic exposure in humans at the maximum recommended human dose (MRHD) of 180 mg to pregnant rats and rabbits, respectively. In an embryofetal development study in rats, bempedoic acid was given orally to pregnant rats at 10, 30, and 60 mg/kg/day during the period of organogenesis from gestation day 6 to 17. There were increases in the incidence of non-adverse fetal skeletal variations (bent long bones and bent scapula and incomplete ossification) at doses ≥ 10 mg/kg/day (less than the clinical exposure) in the absence of maternal toxicity. At maternally toxic doses, bempedoic acid caused decreases in the numbers of viable fetuses, increases in post-implantation loss, and increased total resorptions at 60 mg/kg/day (11 times MRHD) and reduced fetal body weight at ≥ 30 mg/kg/day (4 times the MRHD). No adverse development effects were observed when bempedoic acid was given to pregnant rabbits during the period of organogenesis (gestation day 6 to 18) at doses up to 80 mg/kg/day (12 times MRHD).
In a pre- and post-natal development study in pregnant rats given oral doses of bempedoic acid at 5, 10, 20, 30 and 60 mg/kg/day throughout pregnancy and lactation (gestation day 6 to lactation day 20), there were adverse effects on delivery in the presence of maternal toxicity, including: increases in stillborn pups, reductions in numbers of live pups, pup survival, pup growth and slight delays in learning and memory at ≥ 10 mg/kg/day (at exposures equivalent to the MRHD).
8.2 Lactation
Risk Summary
There is no information regarding the presence of NEXLETOL in human or animal milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. NEXLETOL decreases cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol and may cause harm to the breastfed infant. Because of the potential for serious adverse reactions in a breastfed infant, based on the mechanism of action, advise patients that breastfeeding is not recommended during treatment with NEXLETOL [see Use in Specific Populations (8.1), Clinical Pharmacology (12.1)].
8.4 Pediatric Use
The safety and effectiveness of NEXLETOL have not been established in pediatric patients.
8.5 Geriatric Use
Of the 3,009 adult patients in the primary hyperlipidemia trials of NEXLETOL, 1,753 (58%) were 65 years of age and older, while 478 (16%) were 75 years of age and older.
Of the 13,970 adult patients in the cardiovascular outcomes trial [see Clinical Studies (14.2)], 8,204 (59%) were 65 years of age and older, while 2,107 (15%) were 75 years of age and older.
No overall differences in safety or effectiveness of NEXLETOL have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
No dosage adjustment is necessary in patients with mild or moderate renal impairment. There is limited experience with NEXLETOL in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2), and NEXLETOL has not been studied in patients with end-stage renal disease (ESRD) receiving dialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment is necessary in patients with mild or moderate hepatic impairment (Child-Pugh A or B) [see Clinical Pharmacology (12.3)]. Patients with severe hepatic impairment (Child-Pugh C) have not been studied.
10. Overdosage
There is no clinical experience with NEXLETOL overdose. In the event of an overdosage, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
11. Nexletol Description
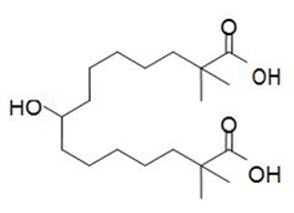
NEXLETOL tablets, for oral use, contain bempedoic acid, an adenosine triphosphate-citrate lyase (ACL) inhibitor. The chemical name for bempedoic acid is 8-hydroxy-2,2,14,14-tetramethyl-pentadecanedioic acid. The molecular formula is C19H36O5, and the molecular weight is 344.5 grams per mole. Bempedoic acid is a white to off-white crystalline powder that is highly soluble in ethanol, isopropanol and pH 8 phosphate buffer, and insoluble in water and aqueous solutions below pH 5.
Structural formula:
Each film-coated tablet of NEXLETOL contains 180 mg of bempedoic acid and the following inactive ingredients: colloidal silicon dioxide, hydroxyl propyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film coating comprises of partially hydrolyzed polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide.
12. Nexletol - Clinical Pharmacology
12.1 Mechanism of Action
Bempedoic acid is an adenosine triphosphate-citrate lyase (ACL) inhibitor that lowers LDL-C by inhibition of cholesterol synthesis in the liver. ACL is an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway. Bempedoic acid and its active metabolite, ESP15228, require coenzyme A (CoA) activation by very long-chain acyl-CoA synthetase 1 (ACSVL1) to ETC-1002-CoA and ESP15228-CoA, respectively. ACSVL1 is expressed primarily in the liver. Inhibition of ACL by ETC-1002-CoA results in decreased cholesterol synthesis in the liver and lowers LDL-C in blood via upregulation of low-density lipoprotein receptors.
12.2 Pharmacodynamics
Administration of bempedoic acid alone, or in combination with other lipid modifying agents, decreases LDL-C, non-high density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (apo B), and total cholesterol (TC) in patients with hyperlipidemia.
12.3 Pharmacokinetics
Bempedoic acid pharmacokinetic parameters are presented as the mean [standard deviation ± (SD)] unless otherwise specified. The steady-state maximum plasma concentration (Cmax) and area under the curve (AUC) following multiple-dose administration of bempedoic acid at 180 mg/day were 20.6 ± 6.1 µg/mL and 289.0 ± 96.4 µg∙h/mL, respectively. Bempedoic acid steady-state pharmacokinetics were generally linear over a range of > 60 mg to 220 mg (approximately 33% to 122% of the recommended dosage of 180 mg daily). There were no time-dependent changes in bempedoic acid pharmacokinetics following repeat administration at the recommended dosage, and bempedoic acid steady-state was achieved after 7 days. The mean accumulation ratio was approximately 2.3-fold.
The steady-state Cmax and AUC of the active metabolite (ESP15228) of bempedoic acid were 2.8 ± 0.9 µg/mL and 51.2 ± 17.2 µg∙h/mL, respectively. ESP15228 likely made a minor contribution to the overall clinical activity of bempedoic acid based on systemic exposure, relative potency, and pharmacokinetic properties.
Absorption
Pharmacokinetic data indicate that bempedoic acid is absorbed with a median time to maximum concentration of 3.5 hours when administered as NEXLETOL 180 mg tablets.
Distribution
The bempedoic acid apparent volume of distribution (V/F) was 18 L. Plasma protein binding of bempedoic acid, its glucuronide and its active metabolite, ESP15228, were 99.3%, 98.8% and 99.2%, respectively. Bempedoic acid does not partition into blood cells.
Elimination
The steady-state clearance (CL/F) of bempedoic acid was 11.2 mL/min after once-daily dosing; renal clearance of unchanged bempedoic acid represented less than 2% of total clearance. The mean ± SD half-life for bempedoic acid in humans was 21 ± 11 hours at steady-state.
Metabolism
The primary route of elimination for bempedoic acid is through metabolism of the acyl glucuronide. Bempedoic acid is also reversibly converted to an active metabolite (ESP15228) based on aldo-keto reductase activity observed in vitro from human liver. Mean plasma AUC metabolite/parent drug ratio for ESP15228 following repeat-dose administration was 18% and remained constant over time. Both compounds are converted to inactive glucuronide conjugates in vitro by UGT2B7. Bempedoic acid, ESP15228 and their respective conjugated forms were detected in plasma with bempedoic acid accounting for the majority (46%) of the AUC0-48h and its glucuronide being the next most prevalent (30%). ESP15228 and its glucuronide represented 10% and 11% of the plasma AUC0-48h, respectively.
Excretion
Following single oral administration of 240 mg of bempedoic acid (1.3 times the approved recommended dose), approximately 70% of the total dose (bempedoic acid and its metabolites) was recovered in urine, primarily as the acyl glucuronide conjugate of bempedoic acid, and approximately 30% was recovered in feces. Less than 5% of the administered dose was excreted as unchanged bempedoic acid in feces and urine combined.
Specific Populations
Patients with Renal Impairment
Pharmacokinetics of bempedoic acid was evaluated in a single-dose pharmacokinetic study in subjects with varying degrees of renal function. The mean bempedoic acid AUC in subjects with mild renal impairment (n = 8) were 1.5-fold higher compared to those with normal renal function (n = 6). Relative to those with normal renal function, mean bempedoic acid AUCs were higher in patients with moderate (n = 5) or severe (n = 5) renal impairment by 2.3-fold and 2.4-fold, respectively.
A population pharmacokinetic analysis was performed on pooled data from all clinical trials (n = 2,261) to further evaluate the effects of renal function on the steady-state AUC of bempedoic acid. Compared to patients with normal renal function, the mean bempedoic acid exposures were higher in patients with mild or moderate renal impairment by 1.4-fold (90% CI: 1.3, 1.4) and 1.9-fold (90% CI: 1.7, 2.0), respectively. These differences were not clinically significant. Clinical studies of NEXLETOL did not include patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) or patients with ESRD on dialysis [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The pharmacokinetics of bempedoic acid and its metabolite (ESP15228) was studied in patients with normal hepatic function or mild or moderate hepatic impairment (Child-Pugh A or B) following a single dose (n = 8/group). Compared to patients with normal hepatic function, the bempedoic acid mean Cmax and AUC were decreased by 11% and 22%, respectively, in patients with mild hepatic impairment and by 14% and 16%, respectively, in patients with moderate hepatic impairment. Compared to patients with normal hepatic function, the ESP15228 mean Cmax and AUC were decreased by 13% and 23%, respectively, in patients with mild hepatic impairment and by 24% and 36%, respectively, in patients with moderate hepatic impairment. This is not expected to result in lower efficacy.
Bempedoic acid was not studied in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Cytochrome P450 Substrates
In vitro metabolic interaction studies suggest that bempedoic acid, as well as its active metabolite and glucuronide forms are not metabolized by and do not interact with cytochrome P450 enzymes.
Transporter-mediated Drug Interactions
In vitro drug interaction studies suggest bempedoic acid, as well as its active metabolite and glucuronide form, are not substrates of commonly characterized drug transporters with the exception of bempedoic acid glucuronide, which is an OAT3 substrate. Bempedoic acid weakly inhibits OAT3 at high multiples of clinically relevant concentrations, and bempedoic acid and its glucuronide weakly inhibit OATP1B1, and OATP1B3 at clinically relevant concentrations. Bempedoic acid weakly inhibits OAT2 in vitro, which is likely the mechanism responsible for minor elevations in serum creatinine and uric acid [see Adverse Reactions (6.1)].
Probenecid
Administration of bempedoic acid 180 mg with steady-state probenecid resulted in a 1.7- and a 1.2-fold increase in bempedoic acid AUC and Cmax, respectively. AUC and Cmax for bempedoic acid active metabolite (ESP15228) were increased 1.9- and 1.5-fold, respectively. These elevations are not clinically meaningful and do not impact dosing recommendations.
Statins
The pharmacokinetic interactions between bempedoic acid (at systemic exposure relevant to the indicated CVD population) and simvastatin 20 mg, atorvastatin 10 mg, pravastatin 40 mg, and rosuvastatin 10 mg were evaluated in clinical trials.
Simvastatin: Administration of simvastatin 20 mg with 240 mg of bempedoic acid or 40 mg with 180 mg of bempedoic acid in healthy subjects at steady-state resulted in approximately 2-fold (91% for 20 mg and 96% for 40 mg) and 1.5-fold (54% for 20 mg and 52% for 40 mg) increases in simvastatin acid AUC and Cmax, respectively [see Drug Interactions (7)].
Pravastatin: Administration of pravastatin 40 mg with steady-state bempedoic acid 240 mg in healthy subjects resulted in 99% (2-fold) and 104% (2-fold) increases in pravastatin acid AUC and Cmax, respectively [see Drug Interactions (7)].
Ezetimibe
Increases in AUC and Cmax for ezetimibe were less than 20% when a single dose of ezetimibe was taken with steady-state bempedoic acid. Total ezetimibe (ezetimibe and its glucuronide form) and ezetimibe glucuronide AUC and Cmax increased approximately 1.6- and 1.8-fold, respectively. These elevations are not clinically meaningful and do not impact dosing recommendations.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Bempedoic acid was negative for mutagenicity in an in vitro Ames assay and negative for clastogenicity in the vitro human lymphocyte chromosome aberration assay. Bempedoic acid was negative in both in vivo mouse micronucleus and in vivo rat bone marrow micronucleus/liver comet assay. In a 2-year rat carcinogenicity study, Wistar rats were given oral doses of bempedoic acid at 3, 10 and 30 mg/kg/day. An increased incidence of liver hepatocellular adenomas and hepatocellular adenomas combined with carcinomas, thyroid gland follicular cell adenoma and follicular cell adenomas combined with carcinomas, and pancreatic islet cell adenomas combined with carcinomas were observed in male rats at the dose of 30 mg/kg/day (exposure equivalent to the maximum recommended human dose (MRHD), based on AUC). In a 2-year mice carcinogenicity study, CD-1 mice were given oral doses of bempedoic acid at 25, 75 and 150 mg/kg/day. Bempedoic acid-related increases in the incidence of liver hepatocellular adenomas, hepatocellular carcinomas and hepatocellular adenomas combined with carcinomas in male mice were observed at 75 and 150 mg/kg/day (exposures equivalent to the MRHD). Observations of liver and thyroid tumors are consistent with PPAR alpha agonism in rodents. The human relevance of pancreatic islet cell tumor findings is unknown.
In fertility and early embryofetal development study in rats, bempedoic acid was given orally to male and female rats at 10, 30 and 60 mg/kg/day. Males were dosed for 28 days prior to mating and females were dosed 14 days prior to mating through gestation day 7. No adverse effects on fertility were observed in females in the absence of maternal toxicity. No effects were observed on male fertility outcomes, but decreases in sperm counts were observed at 60 mg/kg/day (9 times the MRHD).
14. Clinical Studies
14.1 Cardiovascular Outcomes Trial in Adults With CVD or at High Risk for CVD
Trial 1 (NCT02993406) was a randomized, double-blind, placebo-controlled, event-driven trial in 13,970 adult patients with established CVD (70%) or at high risk for a CVD event but without CVD (30%) who were not receiving recommended statin dosages. Patients with established CVD had documented history of coronary artery disease, symptomatic peripheral arterial disease, and/or cerebrovascular atherosclerotic disease. Patients without established CVD were considered at high risk for CVD based on meeting at least one of the following criteria:
- (1)
- Diabetes mellitus (type 1 or type 2) in females over 65 years of age or males over 60 years of age;
- (2)
- A Reynolds Risk score > 30% or a SCORE Risk score > 7.5% over 10 years. Reynolds risk score and SCORE risk score evaluate a 10-year risk of having a cardiovascular (CV) event. The Reynolds risk score is based on the following risk factors: sex, age, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, high sensitivity C-reactive protein (hsCRP), and familial history of CVD events. LDL-C is an additional risk factor considered in SCORE risk score; or
- (3)
- A coronary artery calcium score >400 Agatston units at any time in the past.
Patients were randomized 1:1 to receive either oral NEXLETOL 180 mg per day (n = 6,992) or placebo (n = 6,978), alone or as an add on to other background lipid-lowering therapies. Background therapy could include less than low-intensity statin dosages. Overall, 95.3% of adult patients were followed until the end of the trial or death. The median follow-up duration was 3.4 years.
Baseline Demographics and Disease Characteristics
At baseline, the mean age was 66 years (range 21 to 92 years), 59% were 65 years of age and older, 15% were 75 years of age and older, 48% were female, 91% were White, 2% were Black or African American, 4% were American Indian or Alaska Native, 2% were Asian, and 1% were other races; 17% identified as Hispanic or Latino ethnicity.
Selected additional baseline characteristics included hypertension (85%), diabetes mellitus (46%), current tobacco user (22%), eGFR < 60 mL/min per 1.73 m2 (21%), and a mean body mass index of 30 kg/m2. The mean baseline LDL-C was 139 mg/dL. At baseline, 38% of patients were taking at least one lipid-modifying therapy, including less than low-intensity statin dosages (23%), ezetimibe (12%), or fibrates (5%). Most patients were taking at least one other CV medication including acetylsalicylic acid (57%), selective beta blockers (52%), angiotensin converting enzyme inhibitors (40%), or angiotensin receptor blockers (32%).
Efficacy Results
The risk for the primary composite endpoint (MACE-4: time to first occurrence of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization; p= 0.0037) and the key secondary composite endpoint (MACE-3: time to first occurrence of CV death, nonfatal myocardial infarction, or nonfatal stroke; p= 0.0058) was significantly reduced in NEXLETOL-treated patients compared to placebo-treated patients (see Table 4). The difference between the NEXLETOL and placebo groups in mean percent change in LDL-C from baseline to Month 6 was -20% (95% CI: -21%, -19%).
| NEXLETOL N=6,992 | Placebo N=6,978 | NEXLETOL vs. Placebo | |
|---|---|---|---|
| Endpoint | n (%) | n (%) | Hazard Ratio (95% CI) |
| CI = confidence interval; MACE = major adverse cardiac event. aHazard ratio and corresponding 95% CI were based on a Cox proportional hazard model fitting treatment as explanatory variable. This table also presents the time to first occurrence for each of the components of MACE-4; patients may have been included in more than one category |
|||
| Primary Composite Endpoint | |||
| Cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization (MACE-4) | 819 (11.7) | 927 (13.3) | 0.87 (0.79, 0.96) |
| Key Secondary Endpoint | |||
| Cardiovascular death, non-fatal myocardial infarction, non-fatal stroke (MACE-3) | 575 (8.2) | 663 (9.5) | 0.85 (0.76, 0.96) |
| Components of Primary Composite Endpoint | |||
| Non-fatal myocardial infarction | 236 (3.4) | 317 (4.5) | 0.73 (0.62, 0.87) |
| Coronary revascularization | 435 (6.2) | 529 (7.6) | 0.81 (0.72, 0.92) |
| Non-fatal stroke | 119 (1.7) | 144 (2.1) | 0.82 (0.64, 1.05) |
| Cardiovascular death | 269 (3.8) | 257 (3.7) | 1.04 (0.88, 1.24) |
The Kaplan-Meier estimates of the cumulative incidence of the MACE-4 and MACE-3 endpoints are shown in Figure 1 and 2 below.
Figure 1: Cumulative Incidence of Primary Composite Endpoint (MACE-4) Over 4.5 Years in Adults with Established CVD or at High Risk for CVD (Trial 1)
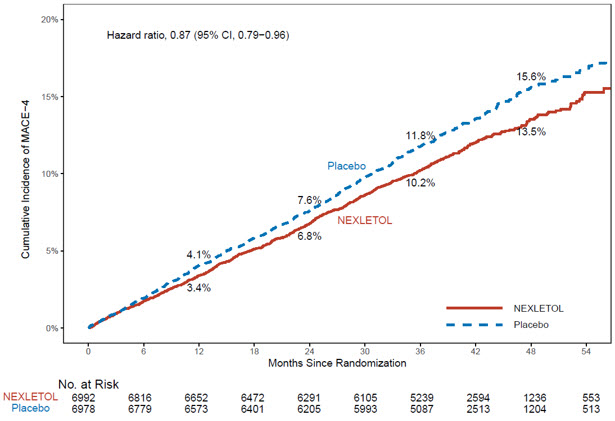
MACE = major adverse cardiac event
MACE-4 was defined as the time to first occurrence of the composite endpoint of CV death, nonfatal MI, nonfatal stroke, or coronary revascularization.
Figure 2: Cumulative Incidence of Composite Endpoint (MACE-3) Over 4.5 Years in Adults with Established CVD or at High Risk for CVD (Trial 1)
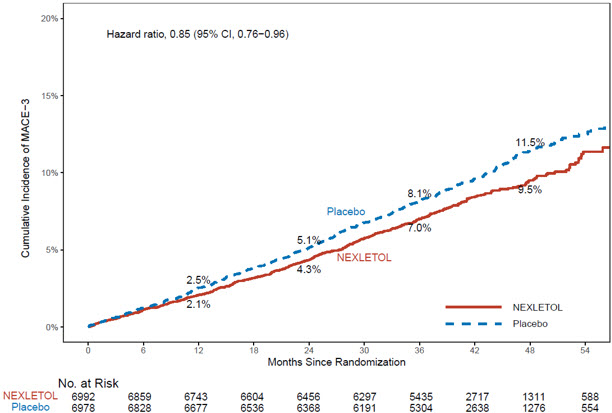
MACE = major adverse cardiac event
MACE-3 was defined as time to first occurrence of the composite endpoint of CV death, nonfatal MI, or nonfatal stroke.
14.2 Primary Hyperlipidemia Trials in Adults
The efficacy of NEXLETOL as an adjunct to diet and statin therapy, to reduce elevated LDL-C in adults with primary hyperlipidemia (including HeFH) was investigated in two multi-center, randomized, double-blind, placebo-controlled trials that enrolled 3,009 adult patients with HeFH or established CVD who were on maximally tolerated statin therapy (Trials 2 and 3). Demographics and baseline disease characteristics were balanced between the treatment arms in these trials. In both trials, the maximum LDL-C lowering effects occurred at Week 4. These results were consistent across all subgroups studied in any of the trials, including age, sex, race, ethnicity, region, history of diabetes, baseline LDL-C, body mass index (BMI), HeFH status, and background therapies.
Trial 2 (NCT02666664)
Trial 2 was a multi-center, randomized, double-blind, placebo-controlled, primary hyperlipidemia (52-week) trial in adult patients with HeFH and/or CVD. Efficacy of NEXLETOL was evaluated at Week 12. The trial included 2,230 patients randomized 2:1 to receive either oral NEXLETOL (n = 1,488) or placebo (n = 742) as add-on to a maximally tolerated lipid-lowering therapy. Maximally tolerated lipid-lowering therapy was defined as a maximally tolerated statin dose alone or in combination with other lipid-lowering therapies. Patients were stratified by presence of HeFH and by baseline statin intensity. Patients on simvastatin 40 mg per day or higher and patients taking PCSK9 inhibitors were excluded from the trial.
Baseline Demographics and Disease Characteristics: Overall, the mean age at baseline was 66 years (range: 24 to 88 years), 61% were 65 years of age and older, 27% were female, 96% were White, 3% were Black or African American , and 1% were Asian; 2% identified as Hispanic or Latino ethnicity. Ninety-five percent (95%) of patients had established CVD, and 5% of patients had HeFH. Twenty-nine percent (29%) of patients had diabetes at baseline. The mean baseline LDL-C was 103.2 mg/dL. At the time of randomization, all patients were receiving statin therapy and 50% were receiving high-intensity statin therapy.
Efficacy Results: The primary efficacy outcome measure of the trial was the percent change from baseline to Week 12 in LDL-C. The difference between the NEXLETOL and placebo groups in mean percent change in LDL-C from baseline to Week 12 was -18% (95% CI: -20%, -16%; p < 0.001). High-density lipoprotein (HDL) and triglycerides (TG) were examined as exploratory endpoints and were not included in the statistical hierarchy. The difference between the NEXLETOL and placebo groups in mean percent change from baseline to Week 12 was -6% for HDL and median percent change from baseline to Week 12 was +3% for TG. For additional results see Table 4 and Figure 3.
| LDL-C*,† | Non-HDL-C† | apo B† | TC† | |
|---|---|---|---|---|
| apo B = apolipoprotein B; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol. | ||||
| Background statin: atorvastatin, simvastatin, pravastatin, and/or other lipid-lowering therapies | ||||
|
||||
| NEXLETOL (180 mg/day; n = 1488‡) | -17 | -12 | -9 | -10 |
| Placebo (n = 742‡) | 2 | 2 | 3 | 1 |
| Mean Difference from Placebo (95% CI) | -18 (-20, -16) | -13 (-15, -12) | -12 (-14, -10) | -11 (-13, -10) |
Trial 3 (NCT02991118)
Trial 3 was a multi-center, randomized, double-blind, placebo-controlled, primary hyperlipidemia (52-week) trial in patients with HeFH and/or CVD. Efficacy of NEXLETOL was evaluated at Week 12. The trial included 779 patients randomized 2:1 to receive either oral NEXLETOL (n = 522) or placebo (n = 257) as add-on to a maximally tolerated lipid-lowering therapy. Maximally tolerated lipid-lowering therapy was defined as a maximally tolerated statin dose alone or in combination with other lipid-lowering therapies. Patients were stratified by presence of HeFH and baseline statin intensity. Patients on simvastatin 40 mg/day or higher were excluded from the trial.
Baseline Demographics and Disease Characteristics: Overall, the mean age at baseline was 64 years (range: 28 to 91 years), 51% were 65 years of age and older, 36% were female, 94% were White, 5% were Black or African American, and 1% were Asian; 8% identified as Hispanic or Latino ethnicity. Ninety-five percent (95%) of patients had established CVD, and 5% of patients had HeFH. Thirty percent (30%) of patients had diabetes at baseline. The mean baseline LDL-C was 120.4 mg/dL. At the time of randomization, 90% of patients were receiving statin therapy, 53% were receiving high-intensity statin therapy, and 0.3% were receiving PCSK9 inhibitors.
Efficacy Results: The primary efficacy outcome measure of the trial was the percent change from baseline to Week 12 in LDL-C. The difference between the NEXLETOL and placebo groups in mean percent change in LDL-C from baseline to Week 12 was -17% (95% CI: -21%, -14%; p < 0.001). HDL and TG were exploratory endpoints and not included in the statistical hierarchy. The difference between the NEXLETOL and placebo groups in mean percent change from baseline to Week 12 was -6% for HDL and the median percent change from baseline was -2% for TG. For additional results see Table 5 and Figure 3.
| LDL-C*,† | Non-HDL-C† | apo B† | TC† | |
|---|---|---|---|---|
| apo B = apolipoprotein B; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol. | ||||
| Background statin: atorvastatin, simvastatin, rosuvastatin, pravastatin, fluvastatin, pitavastatin, and lovastatin ± other lipid-lowering therapies | ||||
|
||||
| NEXLETOL (180 mg/day; n = 522‡) | -15 | -11 | -9 | -10 |
| Placebo (n = 257‡) | 2 | 2 | 4 | 1 |
| Difference from Placebo (95% CI) | -17 (-21, -14) | -13 (-16, -10) | -13 (-16, -10) | -11 (-14, -9) |
Figure 3: Mean Percent Change from Baseline in LDL-C Over 52 Weeks in Adult Patients with HeFH and/or CVD on Maximally Tolerated Statin Treated with NEXLETOL or Placebo (Trial 2 and Trial 3)
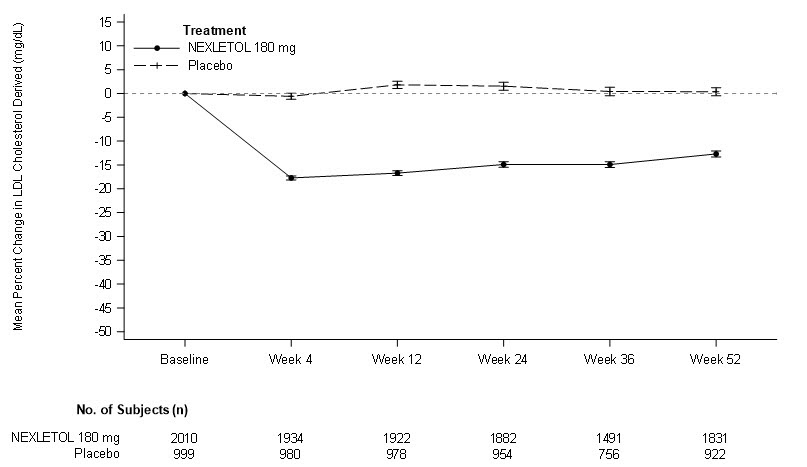
LDL-C derived is calculated from the Friedewald equation: LDL-C = TC - HDL-C - TG/5 in mg/dL.
The error bars represent standard error.
16. How is Nexletol supplied
How Supplied
NEXLETOL tablets are supplied as follows:
| Tablet Strength | Description | Package Configuration | NDC No. |
|---|---|---|---|
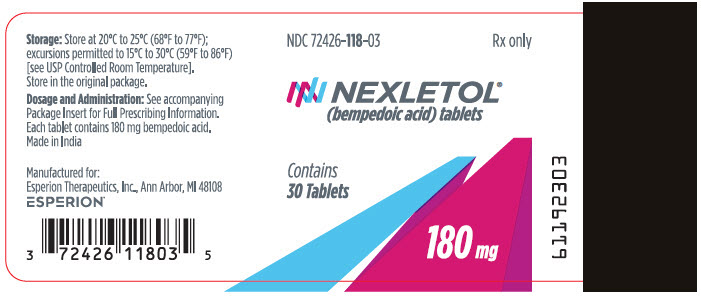
| 180 mg | White to off white and oval, debossed with "180" on one side and "ESP" on the other side | Bottle of 30 tablets with child-resistant cap | 72426-118-03 |
| Bottle of 90 tablets with child-resistant cap | 72426-118-09 |
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Patient Information).
Risk of Hyperuricemia
Advise patients of the risk of elevated serum uric acid levels, including development of gout. Inform patients that serum uric acid levels may be monitored during treatment with NEXLETOL. Patients with signs or symptoms of hyperuricemia should contact their healthcare provider if symptoms occur [See Warnings and Precautions (5.1)]
Risk of Tendon Rupture
Inform patients of the risk of tendon rupture. Advise patients to rest at the first sign of tendinitis or tendon rupture and to immediately contact their healthcare provider if tendinitis or tendon rupture symptoms occur [see Warnings and Precautions (5.2)].
Risk of Myopathy with Concomitant Use of Simvastatin or Pravastatin
Advise patients to notify their healthcare provider(s) if they are taking, or plan to take simvastatin or pravastatin. The risk of myopathy occurring with the use of simvastatin or pravastatin may be increased when taken with NEXLETOL [see Drug Interactions (7)].
Pregnancy
Advise pregnant women of the potential risk to a fetus based on NEXLETOL's mechanism of action. Advise females to inform their healthcare provider of a known or suspected pregnancy. Advise patients that there is a pregnancy safety study that monitors pregnancy outcomes in patients exposed to NEXLETOL during pregnancy. Encourage these patients to report their pregnancy to Esperion at 1-833-377-7633 [see Use in Specific Populations (8.1)].
Manufactured for:
Esperion Therapeutics, Inc.
3891 Ranchero Drive, Suite 150
Ann Arbor, MI 48108
NEXLETOL® (bempedoic acid) tablets
© 2024 Esperion Therapeutics, Inc.
| PATIENT INFORMATION NEXLETOL® (NEX-le-tol) (bempedoic acid) tablets, for oral use |
|||||
|---|---|---|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Revised: 03/2024 | ||||
| What is NEXLETOL?
NEXLETOL is a prescription medicine used:
|
|||||
| Do not take NEXLETOL if you are allergic to bempedoic acid or any of the ingredients in NEXLETOL. See the end of this leaflet for a complete list of ingredients in NEXLETOL. Stop taking NEXLETOL and call your healthcare provider or go to the nearest hospital emergency room right away if you have any signs or symptoms of an allergic reaction including: | |||||
|
|
||||
Before you start taking NEXLETOL, tell your healthcare provider about all your medical conditions, including if you:
Especially tell your healthcare provider if you take or plan to take simvastatin or pravastatin (other cholesterol-lowering medicines). Taking simvastatin or pravastatin with NEXLETOL may increase your risk of developing muscle pain or weakness (myopathy). Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|||||
How should I take NEXLETOL?
|
|||||
| What are possible side effects of NEXLETOL? NEXLETOL may cause serious side effects, including:
|
|||||
|
|
||||
|
|||||
|
|
||||
|
|||||
| The most common side effects of NEXLETOL in people with primary hyperlipidemia include: | |||||
|
|
||||
| The most common side effects of NEXLETOL in people with heart problems include: | |||||
|
|
||||
| Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of NEXLETOL. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||
How should I store NEXLETOL?
|
|||||
| General information about the safe and effective use of NEXLETOL.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NEXLETOL for a condition for which it was not prescribed. Do not give NEXLETOL to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NEXLETOL that is written for healthcare professionals. |
|||||
What are the ingredients in NEXLETOL?
|
|||||
| Manufactured for: Esperion Therapeutics, Inc. 3891 Ranchero Drive, Suite 150 Ann Arbor, MI 48108 © 2024 Esperion Therapeutics, Inc. |
|||||
| NEXLETOL
bempedoic acid tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Esperion Therapeutics, Inc. (029516312) |
More about Nexletol (bempedoic acid)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (7)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- FDA approval history
- Drug class: miscellaneous antihyperlipidemic agents
- Breastfeeding
- En español