MD-76R: Package Insert / Prescribing Info
Package insert / product label
Generic name: diatrizoate meglumine and diatrizoate sodium
Dosage form: injection
Drug class: Ionic iodinated contrast media
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
MD-76R Description
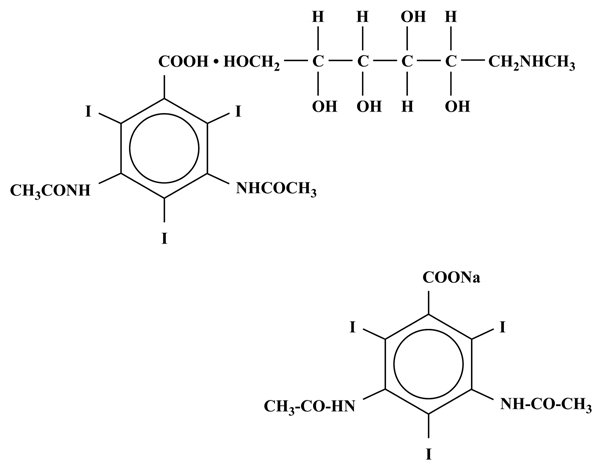
MD-76R (Diatrizoate Meglumine and Diatrizoate Sodium Injection USP) is a radiopaque contrast agent supplied as a sterile, aqueous solution. Intended for intravascular administration, MD-76R contains 66% w/v 1-deoxy-1-(methylamino)-D-glucitol 3,5-diacetamido-2,4,6, triiodobenzoate (salt) and 10% w/v monosodium 3,5-diacetamido-2,4,6, triiodobenzoate. The two salts have the following structural formulae:
Each mL provides 660 mg diatrizoate meglumine and 100 mg diatrizoate sodium, 0.125 mg monobasic sodium phosphate as a buffer and 0.11 mg edetate calcium disodium as a sequestering agent. The pH has been adjusted between 6.5 to 7.7 with either a meglumine and sodium hydroxide combination, or diatrizoic acid. Each mL contains approximately 3.65 mg (0.16 mEq) sodium and 370 mg of organically bound iodine. The viscosity of the solution is 16.4 cps at 25°C and 10.5 cps at 37°C. It is hypertonic to blood with an osmolality of 1551 m0sm/Kg. At the time of manufacture, the air in the container is replaced by nitrogen.
MD-76R - Clinical Pharmacology
Following intravascular injection, MD-76R is rapidly transported through the bloodstream to the kidneys and is excreted unchanged in the urine by glomerular filtration. The pharmacokinetics of intravascularly administered radiopaque contrast media are usually best described by a two compartment model with a rapid alpha phase for drug distribution and a slower beta phase for drug elimination. In patients with normal renal function, the alpha and beta half-lives of MD-76R were approximately 10 and 100 minutes, respectively.
Renal accumulation is sufficiently rapid that the period of maximal opacification of the renal passages may begin as early as 5 minutes after injection. In infants and small children, excretion takes place somewhat more promptly than in adults, so that maximal opacification occurs more rapidly and is less sustained. The normal kidney eliminates the contrast medium almost immediately. In nephropathic conditions, particularly when excretory capacity has been altered, the rate of excretion varies unpredictably, and opacification may be delayed for 30 minutes or more after injection; with severe impairment, opacification may not occur. Generally, however, the medium is concentrated in sufficient amounts and promptly enough to permit a thorough evaluation of the anatomy and physiology of the urinary tract. Intravascular injection also opacifies those vessels in the path of flow of the medium, permitting visualization until the circulating blood dilutes the concentration of the medium. Thus, selective angiography may be performed following injection directly into veins or arteries.
Injectable iodinated contrast agents are excreted either through the kidneys or through the liver. These two excretory pathways are not mutually exclusive, but the main route of excretion seems to be related to the affinity of the contrast medium for serum albumin. Diatrizoate salts are poorly bound to serum albumin, and are excreted mainly through the kidneys.
The liver and small intestine provide the major alternate route of excretion. In patients with severe renal impairment, the excretion of this contrast medium through the gallbladder and into the small intestine sharply increases.
Diatrizoate salts cross the placental barrier in humans and are excreted in human milk.
CT Scanning of the Head
When used for contrast enhancement in computed tomographic brain scanning, the degree of enhancement is directly related to the amount of iodine administered. Rapid injection of the entire dose yields peak blood iodine concentrations immediately following the injection, which fall rapidly over the next five to ten minutes. This can be accounted for by the dilution in the vascular and extracellular fluid compartments, which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached by about ten minutes; thereafter, the fall becomes exponential. Maximum contrast enhancement frequently occurs after peak blood iodine levels are reached. The delay in maximum contrast enhancement can range from five to forty minutes, depending on the peak iodine levels achieved and the cell type of the lesion. This lag suggests that the contrast enhancement of the image is at least in part dependent on the accumulation of iodine within the lesion and outside the blood pool.
In brain scanning, the contrast medium (MD-76R) does not accumulate in normal brain tissue due to the presence of the “blood brain barrier”. The increase in x-ray absorption in the normal brain is due to the presence of the contrast agent within the blood pool. A break in the blood brain barrier, such as occurs in malignant tumors of the brain, allows accumulation of contrast medium within the interstitial tumor tissue; adjacent normal brain tissue does not contain the contrast medium.
The image enhancement of non-tumoral lesions, such as arteriovenous malformations and aneurysms, is dependent on the iodine content of the circulating blood pool.
CT Scanning of the Body1
In non-neural tissues (during CT of the body), MD-76R diffuses rapidly from the vascular to the extra-vascular space. Increase in x-ray absorption is related to blood flow, concentration of the contrast medium and extraction of the contrast medium by interstitial tissue, since no barrier exists; contrast enhancement is thus due to the relative differences in extra-vascular diffusion between normal and abnormal tissue, a situation quite different than that in the brain.
The pharmacokinetics of MD-76R in normal and abnormal tissues has been shown to be variable.
Enhancement of CT with MD-76R may be of benefit in establishing diagnoses of certain lesions in some sites with greater assurance than is possible with unenhanced CT and in supplying additional features of the lesions. In other cases, the contrast medium may allow visualization of lesions not seen with CT alone or may help to define suspicious lesions seen with unenhanced CT.
Contrast enhancement appears to be greatest within the 30 to 90 seconds after bolus administration of the contrast agent, and after intra-arterial, rather than intravenous, administration. Therefore, the use of a continuous scanning technique (a series of 2 to 3 second scans beginning at the injection-dynamic CT scanning) may improve enhancement and diagnostic assessment of tumors and other lesions, occasionally revealing more extensive disease. A cyst, or similar non-vascularized lesion, may be distinguished from vascularized solid lesions by comparing enhanced and unenhanced scans; non-vascularized lesions show no change in CT number, whereas vascularized lesions would show an increase. The latter might be benign, malignant or normal, but it is unlikely that it would be a cyst, hematoma, or other non-vascularized lesion.
Because unenhanced scanning may provide adequate information in the individual patient, the decision to employ contrast enhancement, which is associated with additional risk and increased radiation exposure, should be based upon a careful evaluation of clinical, other radiological, and unenhanced CT findings.
- 1
-
Young, S. W., Turner, R. J., Castellino, R. A.: “A strategy for the contrast enhancement of malignant tumors using dynamic computer tomography and intravascular pharmacokinetics” Radiology, 137:137-147, October 1980.
Indications and Usage for MD-76R
MD-76R is indicated in excretion urography, aortography, pediatric angiocardiography, peripheral arteriography, selective renal arteriography, selective visceral arteriography, selective coronary arteriography with or without left ventriculography, contrast enhancement of computed tomographic brain imaging and for intravenous digital subtraction angiography.
MD-76R is also indicated for the contrast enhancement in body computed tomography (see CLINICAL PHARMACOLOGY). Continuous or multiple scans separated by intervals of 1 to 3 seconds during the first 30 to 90 seconds post-injection of the contrast medium (dynamic CT scanning) provide enhancement of diagnostic significance. Subsets of patients in whom delayed body CT scans might be helpful have not been identified. Inconsistent results have been reported and abnormal and normal tissues may be isodense during the time frame used for delayed CT scanning. The risks of such indiscriminate use of contrast media are well known and such use is not recommended. At present, consistent results have been documented using dynamic CT techniques only.
Contraindications
MD-76R should not be used for myelography.
Refer to PRECAUTIONS, General concerning hypersensitivity.
Warnings
SEVERE ADVERSE EVENTS - INADVERTENT INTRATHECAL ADMINISTRATION: Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to ensure that this drug product is not administered intrathecally.
Ionic iodinated contrast media inhibit blood coagulation, in vitro, more than nonionic contrast media. Nonetheless, it is prudent to avoid prolonged contact of blood with syringes containing ionic contrast media. Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state, and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended, including close attention to guidewire and catheter manipulation, use of manifold systems and/or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease, but not eliminate, the likelihood of in vitro clotting.
Serious or fatal reactions have been associated with the administration of iodine containing radiopaque media. It is of utmost importance to be completely prepared to treat any contrast medium reaction.
Serious neurologic sequelae, including permanent paralysis, have been reported following injections of concentrated contrast media into arteries supplying the spinal cord. The injection of a contrast medium should never be made following the administration of vasopressors, since they strongly potentiate neurologic effects (see PRECAUTIONS pertaining to Aortography).
In patients with subarachnoid hemorrhage, a rare association between contrast administration and clinical deterioration, including convulsions and death, has been reported. Therefore, administration of intravascular iodinated ionic contrast media in these patients should be undertaken with caution.
A definite risk exists in the use of intravascular contrast agents in patients who are known to have multiple myeloma. In such instances, there has been anuria resulting in progressive uremia, renal failure and eventually death. Although neither the contrast agent nor dehydration has separately proved to be the cause of anuria in myeloma, it has been speculated that the combination of both may be the causative factor. The risk in myelomatous patients is not a contraindication to the procedures; however, partial dehydration in the preparation of these patients for the examination is not recommended, since this may predispose to the precipitation of myeloma protein in the renal tubules. No form of therapy, including dialysis, has been successful in reversing this effect. Myeloma, which occurs most commonly in persons over age 40, should be considered before intravascular administration of a contrast agent.
Administration of radiopaque materials to patients known or suspected to have pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure and measures for treatment of a hypertensive crisis should be available.
Contrast media have been shown to promote the phenomenon of sickling in individuals who are homozygous for sickle cell disease when the material is injected intravenously or intra-arterially.
In patients with advanced renal disease, iodinated contrast media should be used with caution, and only when the need for the examination dictates, since excretion of the medium may be impaired. Patients with combined renal and hepatic disease, those with severe hypertension or congestive heart failure, and recent renal transplant recipients may present an additional risk.
Renal failure has been reported in patients with liver dysfunction who were given an oral cholecystographic agent followed by an intravascular iodinated radiopaque agent, and also in patients with occult renal disease, notably diabetics and hypertensives. In these classes of patients, there should be no fluid restriction and every attempt should be made to maintain normal hydration, prior to contrast medium administration, since dehydration is the single most important factor influencing further renal impairment.
Acute renal failure has been reported in diabetic patients with diabetic nephropathy and in susceptible nondiabetic patients (often elderly with pre-existing renal disease) following the administration of iodinated contrast agents. Therefore, careful consideration of the potential risks should be given before performing this radiographic procedure in these patients.
Caution should be exercised in performing contrast medium studies in patients with endotoxemia and/or those with elevated body temperatures.
Reports of thyroid storm occurring following the intravascular use of iodinated radiopaque agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule suggest that this additional risk be evaluated in such patients before use of this drug.
Serious Cutaneous Adverse Reactions: Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering MD-76R to patients with a history of a severe cutaneous adverse reaction to MD-76R.
Avoid accidental introduction of this preparation into the subarachnoid space, since even small amounts may produce convulsions and possible fatal reactions.
Convulsions have occurred in patients with primary or metastatic cerebral lesions following administration of contrast media for contrast enhancement of CT brain images.
Precautions
General
Diagnostic procedures which involve the use of iodinated intravascular contrast agents should be carried out under the direction of personnel skilled and experienced in the particular procedure to be performed. All procedures utilizing contrast media carry a definite risk of producing adverse reactions. While most reactions may be minor, life threatening and fatal reactions may occur without warning. The risk-benefit factor should always be carefully evaluated before such a procedure is undertaken. A fully equipped emergency cart, or equivalent supplies and equipment, and personnel competent in recognizing and treating adverse reactions of all types should always be available. If a serious reaction should occur, immediately discontinue administration. Since severe delayed reactions have been known to occur, emergency facilities and competent personnel should be available for at least 30 to 60 minutes after administration (see ADVERSE REACTIONS).
Preparatory dehydration is dangerous and may contribute to acute renal failure in infants, young children, the elderly, patients with pre-existing renal insufficiency, patients with advanced vascular disease and diabetic patients.
Severe reactions to contrast media often resemble allergic responses. This has prompted the use of several provocative pretesting methods, none of which can be relied on to predict severe reactions. No conclusive relationship between severe reactions and antigen-antibody reactions or other manifestations of allergy has been established. The possibility of an idiosyncratic reaction in patients who have previously received a contrast medium without ill effect should always be considered. Prior to the injection of any contrast medium, the patient should be questioned to obtain a medical history with emphasis on allergy and hypersensitivity. A positive history of bronchial asthma or allergy (including food), a family history of allergy, or a previous reaction or hypersensitivity to a contrast agent may imply a greater than usual risk. Such a history, by suggesting histamine sensitivity and consequently proneness to reactions, may be more accurate than pretesting in predicting the potential for reactions, although not necessarily the severity or type of reaction in the individual case. A positive history of this type does not arbitrarily contraindicate the use of a contrast agent when a diagnostic procedure is thought essential, but does call for caution (see ADVERSE REACTIONS).
Prophylactic therapy including corticosteroids and antihistamines should be considered for patients who present with a strong allergic history, a previous reaction to a contrast medium, or a positive pretest, since the incidence of reaction in these patients is two to three times that of the general population. Adequate doses of corticosteroids should be started early enough prior to contrast medium injection, and for 24 hours after injection. Antihistamines should be administered within 30 minutes of the contrast medium injection. Recent reports indicate that such pretreatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity. A separate syringe should be used for these injections.
The possibility of thrombosis should be borne in mind when percutaneous techniques are employed.
Consideration must be given to the functional ability of the kidneys before injecting this preparation.
General anesthesia may be indicated in the performance of some procedures in young or uncooperative children and in selected adult patients; however, a higher incidence of adverse reactions has been reported in these patients. This may be attributable to the inability of the patient to identify untoward symptoms, or to the hypotensive effect of anesthesia, which can prolong the circulation time and increase the duration of contact of the contrast agent.
Angiography should be avoided whenever possible in patients with hemocystinuria, because of the risk of inducing thrombosis and embolism.
Information for Patients
Patients receiving iodinated intravascular contrast agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease or known thyroid disease (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food or if you had any reactions to previous injections of dyes used for x-ray procedures (see PRECAUTIONS, General).
- Inform your physician about any other medications you are currently taking, including non-prescription drugs.
- Advise patients to inform their physician if they develop a rash after receiving MD-76R.
Drug/Laboratory Test Interactions
Iodine-containing contrast agents may alter the results of thyroid function tests which depend on iodine estimation, e.g., PBI and radioactive iodine uptake studies. Such tests, if indicated, should be performed prior to the administration of this preparation or delayed for about two weeks following administration.
Contrast agents may interfere with some chemical determinations made on urine specimens; therefore, urine should be collected before administration of the contrast medium, or two or more days afterwards.
Following selective coronary arteriography, transient elevation of creatinine phosphokinase (CPK) has occurred in approximately 30% of patients tested.
Post-arteriographic changes in laboratory studies include transient elevations in BUN, serum creatinine and glucose.
Hypertonic solutions cause a decrease in hematocrit in vitro and in vivo, and shrinkage of red blood cells.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential. However, animal studies suggest that this drug is not mutagenic and does not affect fertility in males or females.
Pregnancy Category B
Diatrizoate sodium and diatrizoate meglumine administered intravenously cross the placenta and are evenly distributed in fetal tissues. No teratogenic effects attributable to diatrizoate sodium or diatrizoate meglumine have been observed in teratology studies performed in animals. There are, however, no adequate and well-controlled studies in pregnant women. Because animal teratology studies are not always predictive of human response, this agent should be used during pregnancy only if clearly needed.
Nursing Mothers
Diatrizoate salts are excreted in human milk. Because of the potential for adverse effects in nursing infants, bottle feedings should be substituted for breast feedings for 24 hours following the administration of this drug.
(Precautions for specific procedures receive comment under that procedure.)
Adverse Reactions/Side Effects
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physio-chemical properties of the contrast media, the dose, and speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, the mode of injection and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
Fatalities have been reported following the administration of iodine-containing contrast agents. Based upon clinical literature, the incidence of death is reported to range from one in 10,000 (0.01 percent) to less than one in 100,000 (0.001 percent).
Nausea, vomiting, flushing, or a generalized feeling of warmth are the reactions seen most frequently with intravascular injection. Symptoms which may occur include chills, fever, sweating, headache, dizziness, pallor, weakness, severe retching and choking, wheezing, a rise or fall in blood pressure, facial or conjunctival petechiae, urticaria, pruritus, rash and other eruptions, edema, cramps, tremors, itching, sneezing and lacrimation. Antihistaminic agents may be of benefit; rarely, such reactions may be severe enough to require discontinuation of dosage.
Although venous tolerance is usually good, there have been reports of a burning or stinging sensation or numbness, venospasm or venous pain, and partial collapse of the injected vein. Neutropenia or thrombophlebitis may occur. Tissue necrosis has occurred with extravasation.
Severe reactions which may require emergency measures may take the form of a cardiovascular reaction characterized by peripheral vasodilatation with resultant hypotension and reflex tachycardia, dyspnea, agitation, confusion, convulsions, and cyanosis progressing to unconsciousness. Or, the histamine-liberating effect of these compounds may induce an allergic-like reaction, which may range in severity from rhinitis or angioneurotic edema to laryngeal or bronchial spasm or anaphylactoid shock. Extremely rare cases of disseminated intravascular coagulation resulting in death have been reported.
Temporary renal shutdown or other nephropathy may occur.
Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration to adult and pediatric patients, including infants. Some patients were treated for hypothyroidism.
Skin and Subcutaneous Tissue Disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)].
In addition to the adverse reactions described above, adverse reactions may sometimes occur as a consequence of the procedure for which the contrast agent is used. Adverse reactions in excretion urography have included cardiac arrest, ventricular fibrillation, anaphylaxis with severe asthmatic reaction, and flushing due to generalized vasodilation. In aortography, the risks of procedures include injury to the aorta and neighboring organs, pleural puncture, renal damage including infarction and acute tubular necrosis with oliguria and anuria, accidental selective filling of the right renal artery during the translumbar procedure in the presence of pre-existent renal disease, retroperitoneal hemorrhage from the translumbar approach, spinal cord injury and pathology associated with the syndrome of transverse myelitis, generalized petechiae, and death following hypotension, arrhythmia, and anaphylactoid reactions. Adverse reactions in pediatric angiocardiography have included arrhythmia and death. During peripheral arteriography, complications have occurred including hemorrhage from the puncture site, thrombosis of the vessel, and brachial plexus palsy following axillary artery injections. During selective coronary arteriography with or without left ventriculography, most patients will have transient ECG changes. Transient arrhythmias may occur infrequently. Ventricular fibrillation may result from manipulation of the catheter during the procedure or administration of the medium. Other reactions may include hypotension, chest pain, and myocardial infarction. Fatalities have been reported. Complications due to the procedure include hemorrhage, thrombosis, pseudoaneurysms at the puncture site, and dislodgment of arteriosclerotic plaques. Dissection of the coronary vessels and transient sinus arrest have occurred rarely.
Adverse reactions in selective renal arteriography include nausea, vomiting, hypotension and hypertension.
Overdosage
Overdosage may occur. The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular system. The symptoms may include cyanosis, bradycardia, acidosis, pulmonary hemorrhage, convulsions, coma and cardiac arrest. Treatment of an overdose is directed toward the support of all vital functions and prompt institution of symptomatic therapy.
Diatrizoate salts are dialyzable.
The intravenous LD50 value of diatrizoate meglumine and diatrizoate sodium (in grams of iodine/kilogram body weight) varied from 5.3 to 6.1 g/kg in mice. The LD50 values decrease as the rate of injection increases.
MD-76R Dosage and Administration
MD-76R should be at body temperature when injected, and may need to be warmed before use. If kept in a syringe for prolonged periods before injection, it should be protected from exposure to strong light.
The patient should be instructed to omit the meal that precedes the examination. Appropriate premedication, which may include a barbiturate, tranquilizer or analgesic drug, may be administered prior to the examination.
A preliminary film is recommended to check the position of the patient and the x-ray exposure factors.
If a minor reaction occurs during administration, the injection should be slowed or stopped until the reaction has subsided. If a major reaction occurs, the injection should be discontinued immediately.
Under no circumstances should either corticosteroids or antihistamines be mixed in the same syringe with the contrast medium because of a potential for chemical incompatibility.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
EXCRETORY UROGRAPHY
Following intravenous injection, MD-76R is rapidly excreted by the kidneys. MD-76R may be visualized in the renal parenchyma 30 seconds following bolus injection. Maximum radiographic density in the calyces and pelves occurs in most instances within 3 to 8 minutes after injection. In patients with severe renal impairment, contrast visualization may be substantially delayed.
Patient Preparation
Appropriate preparation of the patient is important for optimal visualization. A low residue diet is recommended for the day preceding the examination and a laxative is given the evening before the examination, unless contraindicated.
Precautions
In addition to the general precautions previously described, infants and young children should not have any fluid restrictions prior to excretory urography. Injection of MD-76R represents an osmotic load which, if superimposed on increased serum osmolality due to partial dehydration, may magnify hypertonic dehydration (see WARNINGS and PRECAUTIONS, General concerning preparatory dehydration).
Usual Dosage
The dose range for adults is 20 to 40 mL; the usual dose is 20 mL; children require proportionately less. Suggested dosages are as follows:
| under 6 months of age | 4 mL |
| 6 to 12 months | 6 mL |
| 1 to 2 years | 8 mL |
| 2 to 5 years | 10 mL |
| 5 to 7 years | 12 mL |
| 8 to 10 years | 14 mL |
| 11 to 15 years | 16 mL |
In adults, when the smaller dose has provided inadequate visualization, or when poor visualization is anticipated, the 40 mL dose may be given.
The preparation is given by intravenous injection. If flushing or nausea occur during administration, injection should be slowed or briefly interrupted until the side effects have disappeared.
AORTOGRAPHY
MD-76R may be administered intravenously or intra-arterially by accepted techniques to visualize the aorta and its major branches.
Warnings
In addition to the warnings previously described, during aortography by the translumbar technique, extreme care is advised to avoid inadvertent intrathecal injection, since the injection of even small amounts (5 to 7 mL) of the contrast medium may cause convulsions, permanent sequelae, or fatality. Should the accident occur, the patient should be placed upright to confine the hyperbaric solution to a low level, anesthesia may be required to control convulsions, and if there is evidence of a large dose having been administered, a careful cerebrospinal fluid exchange-washout should be considered.
Precautions
In addition to the general precautions previously described, the hazards of aortography include those associated with the particular technique employed, the contrast medium and the underlying pathology which warrants the procedure.
In order to prevent the inadvertent injection of a large dose into a branch of the aorta or intramurally, the position of the catheter tip or needle should be carefully evaluated. A small dose of 1 to 2 mL should be administered to locate the exact site of the needle or catheter tip. Inadvertent direct injection of contrast medium into brachiocephalic vessels may result in significant slowing of heart rate, peripheral hypotension and severe CNS reactions, including convulsions. Toxic effects may also be produced if large quantities of contrast medium are injected directly into aortic branches, such as the renal artery, and repetitive injection of the recommended clinical dosage may be hazardous.
Occasional serious neurologic complications, including paraplegia and quadriplegia, have been reported and may be attributable to an excessive dose being injected into arterial trunks supplying the spinal arteries or to prolonged contact time of the concentrated contrast medium on the CNS tissue. Conditions which can contribute to prolonged contact time include decreased circulation, aortic occlusions distal to the site of injection, abdominal compression, hypotension, general anesthesia or the administration of vasopressors. When these conditions exist or occur, the necessity of performing or continuing the procedure should be carefully evaluated, and the dose and number of repeat injections should be maintained at a minimum, with appropriate intervals between injections.
Severe pain, paresthesia, or peripheral muscle spasm during injection may require discontinuance of the procedure and a reevaluation of the placement of the catheter tip or needle.
Following catheter procedure, gentle pressure hemostasis is advised, followed by observation and immobilization of the limb for several hours to prevent hemorrhage from the site of arterial puncture.
Usual Dosage
For adults and children over 16 years of age, the usual dose is 15 to 40 mL as a single injection, repeated if indicated. Children require less in proportion to weight. Doses up to a total of 160 mL have been given safely.
Since the medium is given by rapid injection in this procedure, patients should be watched for untoward reactions during the injection. Unless general anesthesia is employed, patients should be warned that they may feel some transient pain or burning during the injection, followed by a feeling of warmth immediately afterward.
PEDIATRIC ANGIOCARDIOGRAPHY
Angiocardiography with MD-76R may be performed by injection into a large peripheral vein or by direct catheterization of the heart.
Patient Preparation
Patients should be prepared in a manner similar to that used for intravenous urography. Appropriate preanesthetic medication should be given.
Warning
In addition to the general warnings previously described, the inherent risks of angiocardiography in cyanotic infants and patients with chronic pulmonary emphysema must be weighed against the necessity for performing this procedure. In pediatric angiocardiography, a dose of 10 to 20 mL may be particularly hazardous in infants weighing less than 7 kg. This risk is probably significantly increased if these infants have pre-existing right heart “strain”, right heart failure, and effectively decreased or obliterated pulmonary vascular beds.
Adverse Reactions
In addition to the adverse reactions previously described, clinical studies in man, and related animal experiments, have suggested that the hypertonicity of diatrizoate contrast agents produces significant hemodynamic effects, especially in right-sided injections. Large volumes of such agents cause a drop in peripheral arterial and systemic pressures and cardiac output, a rise in pulmonary arterial and right-heart pressures, bradycardia, and regular ectopic beats. Resulting effects on peripheral arterial and pulmonary arterial pressures are postulated to be due to mechanical blockage of the pulmonary vascular bed and clumping of red cells.
It is suggested that hemodynamic changes be monitored and that pressures considered abnormal under roentgenographic conditions be allowed to return to a pre-angiographic level before continuation of radiopaque injection; this usually takes 15 minutes.
PERIPHERAL ARTERIOGRAPHY
MD-76R may be injected into the peripheral arterial circulation. Injection is made into the femoral or subclavian artery by the percutaneous or operative method.
Patient Preparation
The procedure is normally performed with local or general anesthesia (see PRECAUTIONS, General). Premedication may be employed as indicated.
Precautions
In addition to the general precautions previously described, hypotension or moderate decreases in blood pressure seem to occur frequently with intraarterial (brachial) injections; therefore, the blood pressure should be monitored during the immediate ten minutes after injection; this blood pressure change is transient and usually requires no treatment. Extreme caution during injection of the contrast agent is necessary to avoid extravasation and fluoroscopy is recommended. This is particularly important in patients with severe arterial disease.
Adverse Reactions
In addition to the adverse reactions previously described, since the contrast agent is given by rapid injection, pain and flushing of the skin may occur. Patients not under general anesthesia may experience nausea and vomiting or a transient feeling of warmth. Vascular spasm occurs rarely, as does thrombosis of the vessel and brachial plexus palsy, following axillary artery injection.
SELECTIVE VISCERAL ARTERIOGRAPHY
Usual Dosage
The usual dose for injections into the superior mesenteric artery is 40 mL, with a range of 30 to 60 mL; inferior mesenteric artery, usual dose of 15 mL, with a range of 10 to 25 mL; celiac artery, usual dose of 40 mL, with a range of 30 to 50 mL; hepatic artery, usual dose of 25 mL, with a range of 15 to 35 mL; splenic artery, usual dose of 35 mL, with a range of 30 to 40 mL. These doses may be repeated as necessary.
SELECTIVE CORONARY ARTERIOGRAPHY WITH OR WITHOUT LEFT VENTRICULOGRAPHY
Precautions
In addition to the general precautions previously described, it is recommended that this procedure should not be performed for approximately four weeks following the diagnosis of myocardial infarction. Mandatory prerequisites to the procedure are experienced personnel, ECG monitoring apparatus, and adequate facilities for resuscitation and cardioversion.
Patients should be monitored continuously by ECG throughout the procedure.
Usual Dosage
The usual dosage is 4 to 10 mL injected into either coronary artery and repeated as necessary; doses up to a total of 150 mL have been given. For left ventriculography, the usual dose is 35 to 50 mL injected into the left ventricles and repeated as necessary. The total dose for combined selective coronary arteriography and left ventriculography should not exceed 200 mL.
CONTRAST ENHANCEMENT OF COMPUTED TOMOGRAPHIC (CT) BRAIN IMAGING
Tumors
MD-76R may be useful to enhance the demonstration of the presence and extent of certain malignancies, such as: gliomas including malignant gliomas, glioblastomas, astrocytomas, oligodendrogliomas and gangliomas; ependyomas; medulloblastomas, meningiomas; neuromas; pinealomas; pituitary adenomas; craniopharyngiomas; germinomas; and metastatic lesions.
The usefulness of contrast enhancement for the investigation of the retrobulbar space and in cases of low grade or infiltrative glioma has not been demonstrated.
In cases where lesions have calcified, there is less likelihood of enhancement. Following therapy, tumors may show decreased or no enhancement.
Non-Neoplastic Conditions
The use of MD-76R may be beneficial in the image enhancement of non-neoplastic lesions. Cerebral infarctions of recent onset may be better visualized with the contrast enhancement, while some infarctions are obscured if contrast media are used. The use of iodinated contrast media results in contrast enhancement in about 60% of cerebral infarctions studied from one to four weeks from the onset of symptoms.
Sites of active infection may also be enhanced following contrast medium administration.
Arteriovenous malformations and aneurysms will show contrast enhancement. In the case of these vascular lesions, the enhancement is probably dependent on the iodine content of the circulating blood pool.
The opacification of the inferior vermis following contrast medium administration has resulted in false positive diagnoses in a number of normal studies.
Patient Preparation
No special patient preparation is required for contrast enhancement of CT brain scanning. However, it is advisable to ensure that patients are well hydrated prior to examination.
Usual Dosage
The usual dose is 0.6 mL per pound of body weight (1.3 mL/kg), not to exceed 125 mL, by intravenous administration. In most cases, scanning may be performed immediately after completion of administration. However, when fast scanning equipment (less than 1 minute) is used, consideration should be given to waiting approximately five minutes to allow for maximum contrast enhancement.
CONTRAST ENHANCEMENT IN BODY COMPUTED TOMOGRAPHY1
MD-76R may be administered when necessary to visualize vessels and organs in patients undergoing CT of the chest, abdomen and pelvis.
Patient Preparation
No special patient preparation is required for contrast enhancement in body CT. In patients undergoing abdominal or pelvic examination, opacification of the bowel may be valuable in scan interpretation.
Precautions
In addition to the general precaution previously described, it is advisable to ensure that patients are adequately hydrated prior to examination. Patient motion, including respiration, can markedly affect image quality; therefore, patient cooperation is essential. The use of an intravascular contrast medium can obscure tumors in patients undergoing CT evaluation of the liver resulting in a false negative diagnosis. Dynamic CT scanning is the procedure of choice for malignant tumor enhancement (see CLINICAL PHARMACOLOGY).
Usual Dosage
MD-76R may be administered by intravenous bolus injection, by rapid infusion, or by a combination of both.
For vascular opacification, a bolus injection of 25 to 50 mL may be used, repeated as necessary. When prolonged arterial or venous phase enhancement is required, and for the enhancement of specific lesions, a rapid infusion of 100 mL may be used.
INTRAVENOUS DIGITAL SUBTRACTION ANGIOGRAPHY
Digital subtraction angiography (DSA) is a radiographic modality which allows dynamic imaging of the arterial system following intravenous injection of iodinated x-ray contrast media through the use of image intensification, enhancement of the iodine signal and digital processing of the image data. Temporal subtraction of the images obtained during the “first arterial pass” of the injected contrast medium from images obtained before and after contrast medium injection yield images which are devoid of bone and soft tissue.
Areas that have been most frequently examined by intravenous DSA are the heart, including coronary by-pass grafts; the pulmonary arteries; the arteries of the brachiocephalic circulation; the aortic arch; the abdominal aorta and its major branches including the celiac, mesenterics and renal arteries; the iliac arteries; and the arteries of the extremities.
Patient Preparation
No special patient preparation is required for DSA. However, it is advisable to ensure that patients are well hydrated prior to examination.
Precautions
In addition to the general precautions previously described, the risks associated with DSA are those usually attendant with catheter procedures, and include intramural injections, vessel dissection and tissue extravasation. Small test injections of contrast medium made under fluoroscopic observation to ensure the catheter tip is properly positioned, and in the case of peripheral placement that the vein is of adequate size, will reduce this potential.
Patient motion, including respiration and swallowing, can result in marked image degradation, yielding non-diagnostic studies. Therefore, patient cooperation is essential.
Usual Dosage
MD-76R may be injected either centrally into the superior or inferior vena cava, or peripherally into an appropriate arm vein. For central injections, catheters may be introduced at the antecubital fossa into either the basilic or cephalic vein or at the leg into the femoral vein and advanced to the distal segment of the corresponding vena cava.
For peripheral injections, the catheter is introduced at the antecubital fossa into an appropriate size arm vein. In order to reduce the potential for extravasation during peripheral injection, a catheter of approximately 20 cm in length should be employed.
Depending on the area to be imaged, the usual dose range is 20 to 60 mL. Injections may be repeated as necessary.
Central catheter injections are usually made with a power injector with an injection rate of between 10 and 30 mL/second. When making peripheral injections, rates of 12 to 20 mL/second should be used, depending on the size of the vein. Also, since contrast medium may remain in the arm vein for an extended period following injection, it may be advisable to flush the vein, immediately following injection with an appropriate volume (20 to 25 mL) of 5% Dextrose in water or normal saline.
How is MD-76R supplied
| MD-76®R Glass Vials/Bottles | NDC Number |
| 50x50 mL vials | 0019-1317-15 |
| 12x100 mL bottles | 0019-1317-07 |
| 12x200 mL bottles | 0019-1317-09 |
Storage and Handling
Store below 30°C (86°F). Exposing this product to very cold temperatures may result in crystallization of the salt. If this occurs the container should be brought to room temperature. Shake vigorously to assure complete dissolution of any crystals. The speed of dissolution may be increased by heating with circulating warm air. Before use, examine the product to assure that all solids are redissolved and that the container and closure have not been damaged. This preparation is sensitive to light and must be protected from strong daylight or direct exposure to the sun.
As with all contrast media, containers should be inspected prior to use to ensure that breakage or other damage has not occurred during shipping and handling. All containers should be inspected for closure integrity. Damaged containers should not be used.
References
1Young, S. W., Turner, R. J., Castellino, R. A.: “A strategy for the contrast enhancement of malignant tumors using dynamic computer tomography and intravascular pharmacokinetics” Radiology, 137:137-147, October 1980.
Manufactured by: Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
GBT 13170317
Revised 03/17
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
For Intravascular Use
Sterile Solution
MD-76®R
200 mL
NDC 0019-1317-09
Diatrizoate Meglumine and Diatrizoate Sodium Injection USP
370 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx Only
Protect from light • Store below 30°C (86°F).
Each mL contains 660 mg diatrizoate meglumine and 100 mg diatrizoate sodium, 0.125 mg monobasic sodium phosphate as a buffer and 0.11 mg edetate calcium disodium as a sequestering agent. The pH is adjusted with either a meglumine and sodium hydroxide combination or diatrizoic acid.
Single dose container • Discard unused portion
Usual Dosage: See Package Insert.
11230916
Manufactured by:
Liebel-Flarsheim Company LLC
Raliegh, NC 27616
Made in USA

| MD-76R
diatrizoate meglumine and diatrizoate sodium solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Liebel-Flarsheim Company LLC (057880002) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LIEBEL-FLARSHEIM COMPANY LLC | 109024984 | ANALYSIS(0019-1317) , MANUFACTURE(0019-1317) | |
More about diatrizoate
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (2)
- Side effects
- Dosage information
- During pregnancy
- Drug class: ionic iodinated contrast media
- Breastfeeding
Patient resources
Professional resources
Other brands
Gastrografin, Hypaque, Cystografin, MD-Gastroview, Renografin-60
