Infuvite Adult: Package Insert / Prescribing Info
Package insert / product label
Generic name: ascorbic acid, vitamin A, vitamin D, thiamine, riboflavin, pyridoxine, niacinamide, dexpanthenol, vitamin E, vitamin K1, folic acid, biotin, vitamin B12
Dosage form: injection, solution
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Dec 9, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Nonclinical Toxicology
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
INFUVITE ADULT (multiple vitamins injection), for intravenous use
Initial U.S. Approval: 2003
Indications and Usage for Infuvite Adult
INFUVITE ADULT is a combination of vitamins indicated for prevention of vitamin deficiency in adults and children aged 11 and older receiving parenteral nutrition (1)
Infuvite Adult Dosage and Administration
- •
- INFUVITE ADULT is a combination product that contains the following vitamins: ascorbic acid, vitamin A, vitamin D, thiamine, riboflavin, pyridoxine, niacinamide, dexpanthenol, vitamin E, vitamin K1, folic acid, biotin, and vitamin B12 (2.1)
- •
- INFUVITE ADULT is for administration by intravenous infusion after dilution (2.1)
- •
- Recommended daily dosage is 10 mL (2.1)
- •
- INFUVITE ADULT is supplied as a single dose and as a pharmacy bulk package.
- o
- Single Dose: Add one daily dose of 10 mL (5 mL from Vial 1 and 5 mL from Vial 2) to not less than 500 mL, and preferably 1000 mL, of intravenous dextrose, saline or similar infusion solutions. (2.2)
- o
- Pharmacy Bulk Package: Add the contents of Vial 1 to the contents of Vial 2. This provides ten 10 mL daily doses. Take 10 mL from Vial 2 and add to not less than 500 mL, and preferably 1000 mL, of intravenous dextrose, saline or similar infusion solutions. (2.2)
- •
- After dilution in an intravenous infusion, refrigerate resulting solution unless used immediately. Use solution within 24 hours after dilution (2.2)
- •
- Monitor blood vitamin concentrations (2.3)
- •
- See Full Prescribing Information for drug incompatibilities (2.4)
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- •
- Risk of Aluminum Toxicity: For at risk patients (renal failure or those with prolonged therapy), consider periodic monitoring of aluminum levels (5.1)
- •
- Allergic Reactions: To thiamine may occur (5.2)
- •
- Hypervitaminosis A: Patients with renal failure or liver disease may be at higher risk (5.3)
- •
- Decreased Anticoagulant Effect of Warfarin: Monitor INR (5.4, 7.1)
- •
- Interferes with Megaloblastic Anemia Diagnosis: Avoid during testing for this disorder (5.5)
- •
- Risk of Vitamin Deficiencies or Excesses: Monitor blood vitamin concentrations (5.6)
- •
- False Negative Urine Glucose Tests: Due to vitamin C (5.7)
Adverse Reactions/Side Effects
Adverse reactions have included anaphylaxis and anaphylactoid reactions including shortness of breath, wheezing and angioedema, rash, erythema, pruritis, headache, dizziness, agitation, anxiety, diplopia (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Effect of INFUVITE ADULT on other drugs:
- •
- Antibiotics: Thiamine, riboflavin, pyridoxine, niacinamide, and ascorbic acid decrease activities of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin (7.1)
- •
- Bleomycin: Ascorbic acid and riboflavin may reduce the activity of bleomycin (7.1)
- •
- Levodopa: Pyridoxine may decrease blood levels of levodopa and levodopa efficacy may decrease (7.1)
- •
- Phenytoin: Folic acid may decrease phenytoin blood levels and increase risk of seizure activity (7.1)
- •
- Methotrexate: Folic acid may decrease response to methotrexate (7.1)
Effects of other drugs on INFUVITE ADULT:
- •
- Hydralazine, Isoniazid: Concomitant administration of hydralazine or isoniazid may increase pyridoxine requirements (7.2).
- •
- Phenytoin: May decrease folic acid concentrations (7.2)
- •
- Chloramphenicol: In patients with pernicious anemia, the hematologic response to vitamin B12 therapy may be inhibited (7.2)
Use In Specific Populations
- •
- Pregnancy and Lactation: Pregnant and lactating patients should follow the U.S. Recommended Daily Allowances for their condition, because their vitamin requirements may exceed those of nonpregnant and nonlactating patients (8.1, 8.2)
- •
- Renal Impairment: Monitor renal function, calcium, phosphorus and vitamin A levels in patients with renal impairment (8.6)
- •
- Hepatic Impairment: Monitor vitamin A level in patients with liver disease, high alcohol consumption (8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2024
Full Prescribing Information
1. Indications and Usage for Infuvite Adult
INFUVITE ADULT is a combination of vitamins indicated for the prevention of vitamin deficiency in adults and children aged 11 and older receiving parenteral nutrition.
The physician should not await the development of clinical signs of vitamin deficiency before initiating vitamin therapy.
2. Infuvite Adult Dosage and Administration
2.1 Important Dosage Instructions
INFUVITE ADULT is a combination product that contains the following vitamins: ascorbic acid, vitamin A, vitamin D, thiamine, riboflavin, pyridoxine, niacinamide, dexpanthenol, vitamin E, vitamin K1, folic acid, biotin, and vitamin B12.
INFUVITE ADULT is supplied as a single dose or as a pharmacy bulk package for intravenous use intended for administration by intravenous infusion after dilution.
INFUVITE ADULT Single Dose:
- •
- Provides one daily dose of 10 mL (5 mL of Vial 1 plus 5 mL of Vial 2) which must be diluted prior to intravenous administration [see Dosage and Administration (2.2)].
INFUVITE ADULT PharmacyBulk Package:
- •
- Provides ten 10 mL daily doses when the content of vial 1 is transferred into the content of vial 2. One 10 mL dose is then added directly to intravenous fluid. Pharmacy bulk package of INFUVITE ADULT is intended for dispensing of single doses to multiple patients in a pharmacy admixture program and is restricted to the preparation of admixtures for infusion [see Dosage and Administration (2.2)].
Patients with multiple vitamin deficiencies or with increased vitamin requirements may need multiple daily dosages as indicated. Some patients do not maintain adequate levels of certain vitamins when this formulation in recommended amounts is the only source of vitamins.
2.2 Preparation and Administration Instructions
Do not administer INFUVITE ADULT as a direct, undiluted intravenous injection as it may cause dizziness, faintness, and possible tissue irritation.
INFUVITE ADULT Single Dose:
- •
- Use only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area).
- •
- Add 5 mL of Vial 1 and 5 mL of Vial 2 to at least 500 mL to 1000 mL, of intravenous dextrose or saline solutions.
- •
- Discard unused portion.
- •
- Visually inspect for particulate matter and discoloration prior to administration.
- •
- After INFUVITE ADULT is diluted in an intravenous infusion, refrigerate the resulting solution unless it is to be used immediately, and use the solution within 24 hours after dilution.
- •
- Minimize exposure to light because some of the vitamins in INFUVITE ADULT, particularly A, D and riboflavin, are light sensitive.
INFUVITE ADULT Pharmacy Bulk Package:
- •
- Use only in a suitable work area such as a laminar flow hood (or an equivalent clean air compounding area).
- •
- Transfer the contents of Vial 1 into the contents of Vial 2 to provide ten 10 mL single doses.
- •
- Each bulk vial closure shall be penetrated only one time with a suitable sterile transfer device or dispensing set that allows measured dispensing of the contents.
- •
- Once the closure system has been penetrated, complete dispensing from the pharmacy bulk vial should be completed within 4 hours. The mixed solution may be refrigerated and stored for up to 4 hours.
- •
- Discard unused portion.
- •
- Visually inspect for particulate matter and discoloration prior to administration.
- •
- One daily 10 mL dose should be added directly to at least 500 mL to 1000 mL, of intravenous dextrose, saline or similar infusion solutions.
- •
- After INFUVITE ADULT is diluted in an intravenous infusion, refrigerate the resulting solution unless it is to be used immediately, and use the solution within 24 hours after dilution.
- •
- Minimize exposure to light because some of the vitamins in INFUVITE ADULT, particularly A, D and riboflavin, are light sensitive.
2.3 Monitoring Vitamin Blood Levels
Blood vitamin concentrations should be monitored to ensure maintenance of adequate levels, particularly in patients receiving parenteral multivitamins as the only source of vitamins for long periods of time.
2.4 Drug Incompatibilities
- •
- INFUVITE ADULT is not physically compatible with moderately alkaline solutions such as a sodium bicarbonate solution and other alkaline drugs such as acetazolamide sodium, aminophylline, ampicillin sodium, tetracycline HCl and chlorothiazide sodium.
- •
- Folic acid is unstable in the presence of calcium salts such as calcium gluconate.
- •
- Vitamin A and thiamine in INFUVITE ADULT may react with bisulfite solutions such as sodium bisulfite or vitamin K bisulfate.
- •
- Do not add INFUVITE ADULT directly to intravenous fat emulsions.
- •
- Consult appropriate references for additional listings of physical and chemical compatibility of solutions and drugs with the vitamin infusion when needed. If incompatibilities are identified, avoid admixture or Y-site administration with vitamin solutions.
3. Dosage Forms and Strengths
INFUVITE ADULT Single Dose: is an injection for intravenous administration consisting of two vials labeled Vial 1 (5 mL) and Vial 2 (5 mL). For the vitamin strengths [see Description (11)].
INFUVITE ADULT Pharmacy Bulk Package: is an injection for intravenous administration consisting of two vials labeled Vial 1 (50 mL) and Vial 2 (50 mL Fill in 100 mL Vial). The mixed solution (100 mL) will provide ten 10 mL single doses. For the vitamin strengths [see Description (11)].
Vial 1 (Single Dose and Pharmacy Bulk Package) is supplied as clear, yellow to orange liquid in amber glass vial closed with a rubber stopper, clear aluminum seal and a white flip-off.
Vial 2 (Single Dose and Pharmacy Bulk Package) is supplied as clear, colorless to pale yellow liquid in amber glass vial closed with a rubber stopper, clear aluminum seal and a deep purple flip-off.
4. Contraindications
INFUVITE ADULT is contraindicated in patients who have
- •
- An existing hypervitaminosis, or
- •
- A history of hypersensitivity due to any vitamins or excipients contained in this formulation.
5. Warnings and Precautions
5.1 Aluminum Toxicity
INFUVITE ADULT contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solution, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration. To prevent aluminum toxicity periodically monitor aluminum levels with prolonged parenteral administration of INFUVITE ADULT in patients with renal impairment.
5.2 Allergic Reactions to Thiamine
Allergic reactions such as urticaria, shortness of breath, wheezing and angioedema have been reported following intravenous administration of thiamine, which is found in INFUVITE ADULT. There have been rare reports of anaphylactoid reactions following intravenous doses of thiamine. No fatal anaphylactoid reactions associated with INFUVITE ADULT have been reported.
5.3. Hypervitaminosis A
Hypervitaminosis A, manifested by nausea, vomiting, headache, dizziness, blurred vision has been reported in patients with renal failure receiving 1.5 mg/day retinol and in patients with liver disease, Therefore, supplementation of renal failure patients and patients with liver disease with vitamin A, an ingredient found in INFUVITE ADULT, should be undertaken with caution [see Use in Specific Populations (8.6 and8.7)].
5.4 Decreased Anticoagulant Effect of Warfarin
INFUVITE ADULT contains Vitamin K, which may decrease the anticoagulant action of warfarin. In patients who are on warfarin anticoagulant therapy receiving INFUVITE ADULT monitor blood levels of prothrombin/INR to determine if dose of warfarin needs to be adjusted.
5.5 Interference with Diagnosis of Megaloblastic Anemia
INFUVITE ADULT contains folic acid and cyanocobalamin which can mask serum deficiencies of folic acid and cyanocobalamin in patients with megaloblastic anemia. Avoid the use of INFUVITE ADULT in patients with suspected or diagnosed megaloblastic anemia prior to blood sampling for the detection of the folic acid and cyanocobalamin deficiencies.
5.6 Potential to Develop Vitamin Deficiencies or Excesses
In patients receiving parenteral multivitamins such as with INFUVITE ADULT, blood concentration should be periodically monitored to determine if deficiencies or excesses are developing. INFUVITE ADULT may not correct long-standing specific vitamin deficiencies. The administration of additional therapeutic doses of specific vitamins may be required [see Dosage and Administration (2.3)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other section of the labeling.
- •
- Allergic reactions to thiamine [see Warnings and Precautions (5.2)]
- •
- Hypervitaminosis A [see Warnings and Precautions (5.3)]
The following adverse reactions have been identified during postapproval use of INFUVITE ADULT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic: rash, erythema, pruritis
CNS: headache, dizziness, agitation, anxiety
Ophthalmic: diplopia
7. Drug Interactions
A number of interactions between vitamins and drugs have been reported. The following are examples of these types of interactions:
7.1 Drug Interactions Affecting Co-Administered Drugs
Warfarin: Vitamin K, a component of INFUVITE ADULT, antagonizes the anticoagulant action of warfarin. In patients who are co-administered warfarin and INFUVITE ADULT, blood levels of prothrombin/INR should be monitored to determine if dose of warfarin needs to be adjusted [see Warnings and Precautions (5.4)].
Antibiotics: Thiamine, riboflavin, pyridoxine, niacinamide, and ascorbic acid decrease antibiotic activities of erythromycin, kanamycin, streptomycin, doxycycline, and lincomycin.
Bleomycin: Ascorbic acid and riboflavin inactivate bleomycin in vitro, thus the activity of bleomycin may be reduced.
Levodopa: Pyridoxine may increase the metabolism of levodopa (decrease blood levels of levodopa) and decrease its efficacy.
Phenytoin: Folic acid may increase phenytoin metabolism and lower the serum concentration of phenytoin resulting in increased seizure activity.
Methotrexate: Folic acid may decrease a patient’s response to methotrexate therapy.
7.2 Drug Interactions Affecting Vitamin Levels
Hydralazine, Isoniazid: Concomitant administration of hydralazine or isoniazid may increase pyridoxine requirements.
Phenytoin: Phenytoin may decrease serum folic acid concentrations.
Chloramphenicol: In patients with pernicious anemia, the hematologic response to vitamin B12 therapy may be inhibited by chloramphenicol.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Administration of the approved recommended dose of INFUVITE ADULT in parental nutrition is not expected to cause major birth defects, miscarriage, or adverse maternal or fetal outcomes. Pregnant patients should follow the U.S. Recommended Daily Allowances for pregnancy because their vitamin requirements may exceed those of nonpregnant patients. Deficiency of essential vitamins may result in adverse pregnancy outcomes (see Clinical Considerations). Animal reproduction studies have not been conducted with INFUVITE ADULT.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo-Fetal Risk
Deficiency of essential vitamins has been associated with adverse pregnancy and fetal outcomes, such as maternal folic acid deficiency and an increased risk of neural tube defects. Therefore, parenteral nutrition with multiple vitamins injection should be considered if a pregnant woman’s nutritional requirements cannot be fulfilled by oral or enteral intake.
8.2 Lactation
Risk Summary
Multiple vitamins present in INFUVITE ADULT are also present in human milk. Administration of the approved recommended dose of INFUVITE ADULT In parental nutrition is not expected to cause harm to a breastfed infant. There is no information on the effects of INFUVITE ADULT on milk production. Lactating patients should follow the U.S. Recommended Daily Allowances for their condition, because their vitamin requirements may exceed those of nonlactating patients. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for INFUVITE ADULT and any potential adverse effects on the breastfed child from INFUVITE ADULT or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in children below the age of 11 years have not been established.
8.6 Renal Impairment
INFUVITE ADULT has not been studied in patients with renal impairment. Monitor renal function, calcium, phosphorus and vitamin A levels in patients with renal impairment [see Warnings and Precautions (5.1 and 5.3)].
8.7 Hepatic Impairment
INFUVITE ADULT has not been studied in patients with liver impairments. Monitor vitamin A level in patients with liver disease, high alcohol consumption [see Warnings and Precautions (5.3)].
10. Overdosage
Signs and symptoms of acute or chronic overdosage may be those of individual INFUVITE ADULT component toxicity. In post-marketed surveillance, overdosage with INFUVITE ADULT at two times the prescribed dose did not result in toxicity.
11. Infuvite Adult Description
INFUVITE ADULT (multiple vitamins injection) is a sterile product consisting of two vials provided as a single dose or as a pharmacy bulk package, both intended for intravenous use for administration by intravenous infusion after dilution:
INFUVITE ADULT Single Dose- two 5 mL single-dose vials labeled Vial 1 and Vial 2.
INFUVITE ADULT Pharmacy Bulk Package- two vials – 1 each of Vial 1 (50 mL) and Vial 2 (50 mL Fill in 100 mL Vial). The mixed solution (100 mL) will provide ten 10 mL single doses.
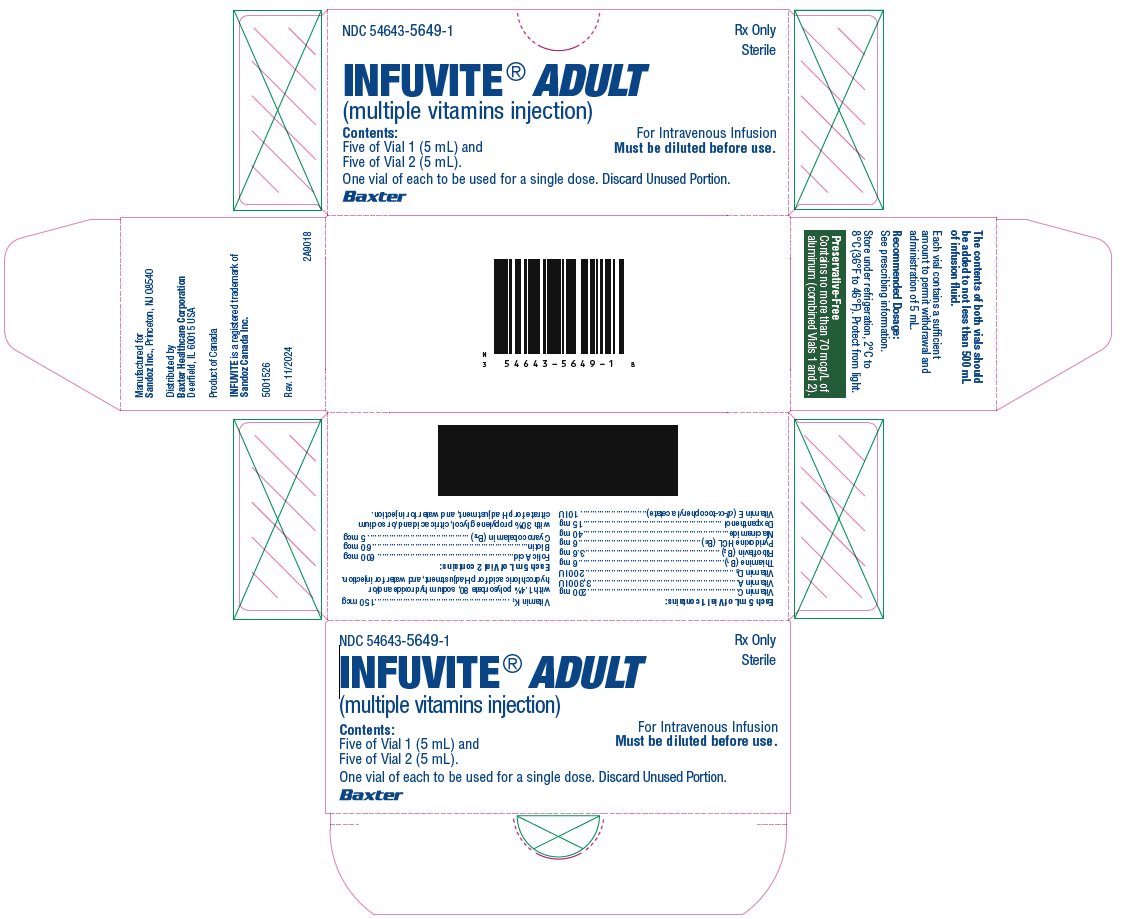
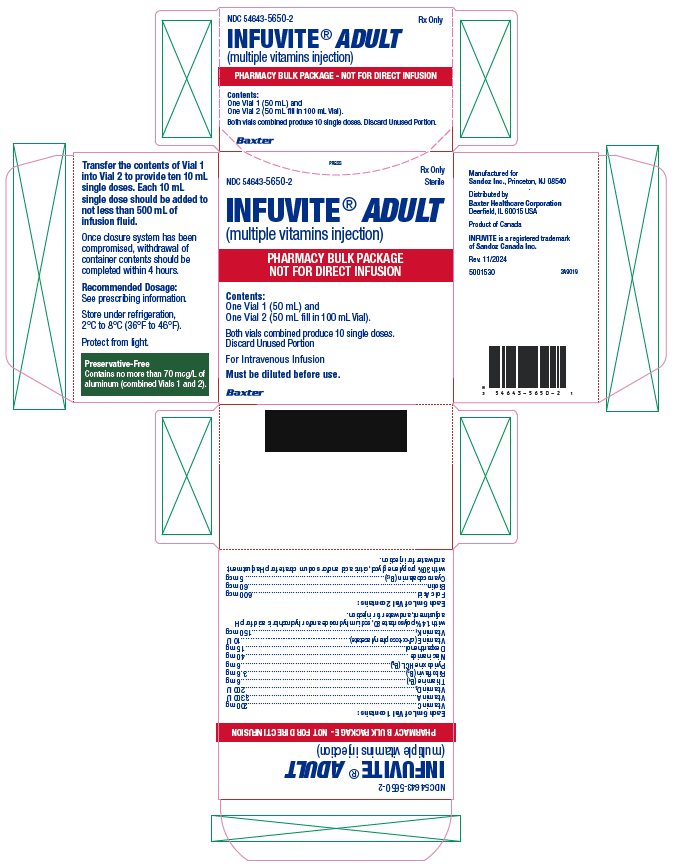
Each 5 mL of Vial 1 contains:
|
Ascorbic acid (Vitamin C) |
200 mg |
|
Vitamin A* (as palmitate) |
3,300 IU |
|
Vitamin D3* (cholecalciferol) |
200 IU |
|
Thiamine (Vitamin B1) (as the hydrochloride) |
6 mg |
|
Riboflavin (Vitamin B2) (as riboflavin 5-phosphate sodium) |
3.6 mg |
|
Pyridoxine HCl (Vitamin B6) |
6 mg |
|
Niacinamide |
40 mg |
|
Dexpanthenol (as d-pantothenyl alcohol) |
15 mg |
|
Vitamin E* (dl-α-tocopheryl acetate) |
10 IU |
|
Vitamin K1* |
150 mcg |
*Polysorbate 80 is used to water solubilize the oil-soluble vitamins A, D, E, and K.
Inactive ingredients: 1.4% polysorbate 80, sodium hydroxide and/or hydrochloric acid for pH adjustment, and water for injection.
Each 5 mL of Vial 2 contains:
|
Folic acid |
600 mcg |
|
Biotin |
60 mcg |
|
Vitamin B12 (cyanocobalamin) |
5 mcg |
Inactive ingredients: 30% propylene glycol, citric acid and/or sodium citrate for pH adjustment, and water for injection.
INFUVITE ADULT makes available a combination of important oil-soluble and water-soluble vitamins in an aqueous solution, formulated for incorporation into intravenous solutions. The liposoluble vitamins A, D, E, and K have been solubilized in an aqueous medium with polysorbate 80, permitting intravenous administration of these vitamins.
Contains no more than 70 mcg/L of aluminum (combined Vials 1 and 2).
16. How is Infuvite Adult supplied
How Supplied
INFUVITE ADULT (multiple vitamins injection) is supplied as follows:
Vial 1 (Single Dose and Pharmacy Bulk Package) is supplied as clear, yellow to orange liquid in amber glass vial closed with a rubber stopper, clear aluminum seal and a white flip-off.
Vial 2 (Single Dose and Pharmacy Bulk Package) is supplied as clear, colorless to pale yellow liquid in amber glass vial closed with a rubber stopper, clear aluminum seal and a deep purple flip-off.
INFUVITE ADULT Single Dose:
|
Carton contains total ten single-dose vials |
NDC 54643-5649-1 |
|
NDC 54643-5657-1 |
|
NDC 54643-5659-1 |
one Vial 1 plus one Vial 2 to be used for a single dose [see Dosage and Administration (2.1)].
INFUVITE ADULT Pharmacy Bulk Package:
|
Carton contains total two vials |
NDC 54643-5650-2 |
|
NDC 54643-5661-2 |
|
NDC 54643-5663-2 |
Mix contents of Vial 1 with Vial 2 to provide 10 single doses [see Dosage and Administration (2.1)].
Storage and Handling
Minimize exposure of INFUVITE ADULT to light because vitamins A, D and riboflavin are light sensitive.
Store under refrigeration, 2°C to 8°C (36°F to 46°F).
17. Patient Counseling Information
Instruct patients (if age appropriate) and caregivers:
- •
- To watch for signs of allergic reactions such as urticaria, shortness of breath, wheezing and angioedema since hypersensitivity reactions may occur to any of the vitamins or excipients contained in INFUVITE ADULT.
- •
- To watch for and immediately report nausea, vomiting, headache, dizziness, blurred vision, especially if patients have renal impairment, as these may be signs of hypervitaminosis A.
- •
- To report other adverse reactions such as rash, erythema, pruritus, headache, dizziness, agitation, anxiety, and diplopia.
- •
- That the patients on warfarin anticoagulant therapy will be monitored periodically with blood prothrombin/ INR levels to determine if their dose of warfarin needs to be adjusted.
- •
- About the significance of periodic monitoring of blood vitamin concentrations to determine if vitamin deficiencies or excesses are developing and the need to monitor renal function, calcium, phosphorus, aluminum and vitamin A levels in patients with renal impairment.
- •
- That INFUVITE ADULT should be avoided in patients with suspected or diagnosed megaloblastic anemia prior to blood sampling for the detection of the folic acid and cyanocobalamin deficiencies.
- •
- That vitamin C (ascorbic acid) contained in INFUVITE ADULT may cause false negative urine glucose results.
Infuvite Adult Single Dose Vials Carton
- NDC 54643-5649-1 Rx Only Sterile
INFUVITE® ADULT (multiple vitamins injection)
Contents:
Five of Vial 1 (5 mL) and
Five of Vial 2 (5 mL).
One vial of each to be used for a single dose. Discard Unused Portion.
For Intravenous Infusion
Must be diluted before use.
Baxter
Infuvite Adult Pharmacy Bulk Vials Carton
- NDC 54643-5650-2 Rx Only Sterile
INFUVITE® ADULT (multiple vitamins injection)
PHARMACY BULK PACKAGE
NOT FOR DIRECT INFUSION
Contents:
One Vial 1 (50 mL) and
One Vial 2 (50 mL fill in 100 mL Vial).
Both vials combined produce 10 single doses. Discard Unused Portion
For Intravenous Infusion
Must be diluted before use.
Baxter
| INFUVITE ADULT
multiple vitamins injection kit |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| INFUVITE ADULT
multiple vitamins injection kit |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Sandoz Inc (005387188) |
Frequently asked questions
More about multivitamin
- Check interactions
- Compare alternatives
- Reviews (242)
- Drug images
- Side effects
- Support group
- Drug class: vitamin and mineral combinations
- En español
Patient resources
Professional resources
- Multivitamins monograph
- Levomefolate, Calcium Acetylcysteine and Mecobalamin Algal (FDA)
- Lexazin Capsules (FDA)
- Multi Vitamin Infusion M.V.I. 12 (FDA)
- Multi Vitamin Infusion M.V.I. Adult (FDA)
Other brands
Nephrocaps, Nephplex Rx, Renal Caps, MVI Adult, ... +13 more