Hibiclens: Package Insert / Prescribing Info
Package insert / product label
Generic name: (chlorhexidine gluconate
Dosage form: topical solution
Drug classes: Antiseptic and germicides, Mouth and throat products
Medically reviewed by Drugs.com. Last updated on Nov 11, 2024.
On This Page
Indications and Usage for Hibiclens
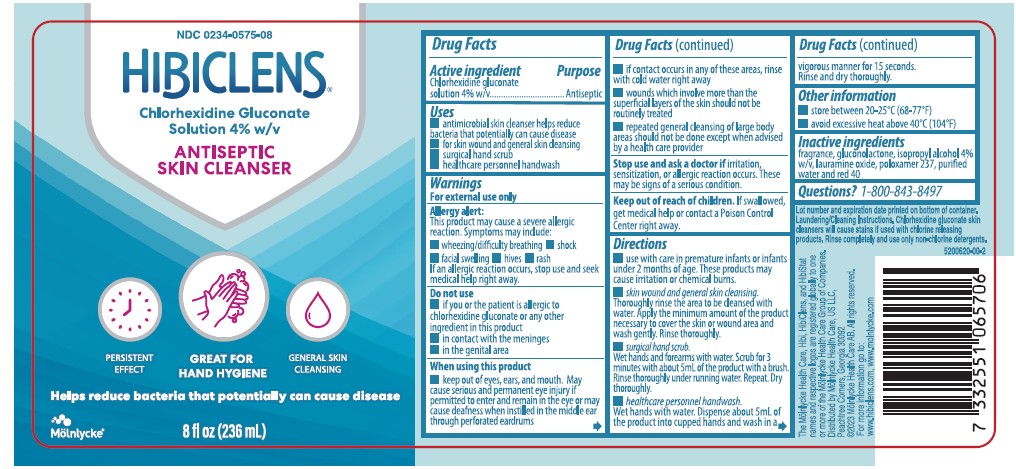
- antimicrobial skin cleanser helps reduce bacteria that potentially can cause disease
- for skin wound and general skin cleansing
- surgical hand scrub
- healthcare personnel handwash
Warnings
For external use only
Allergy alert
This product may cuase a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use
- if you or the patient is allergic to chlorhexidine gluconate or any other ingredient in this product
- in contact with the meninges
- in the genital area
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated eardrums.
- if contact occurs in any of these areas, rinse with cold water right away
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general cleansing of large body areas should not be done except when advised by a health care provider
Stop use and ask doctor if
irritation, sensitization, or allergic reaction occurs. These may be signs of a serious condition.
Hibiclens Dosage and Administration
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- skin wound and general skin cleansing.
Thoroughly rinse the area to be cleansed with water. Apply the minimum amount of the product necessary to cover the skin or wound area and wash gently. Rinse thoroughly.
- surgical hand scrub.
Wet hands and forearms with water. Scrub for 3 minutes with about 5 mL (4 pumps) of the product with a brush. Rinse thoroughly under running water. Repeat. Dry thoroughly.
- healthcare personnel handwash.
Wet hands with water. Dispense about 5 mL (4 pumps) of the product into cupped hands and wash in a vigorous manner for 15 seconds. Rinse and dry thoroughly.
fragrance, gluconolactone, isopropyl alcohol 4% w/v, lauramine oxide, poloxamer 237, purified water and red 40
Laundering and Cleaning Instructions
Chlorhexidine gluconate skin cleansers wil cause stains in used with chlorine releasing products. Rinse completely and use only non-chlorine detergents
Principal Display Panel (Labels)
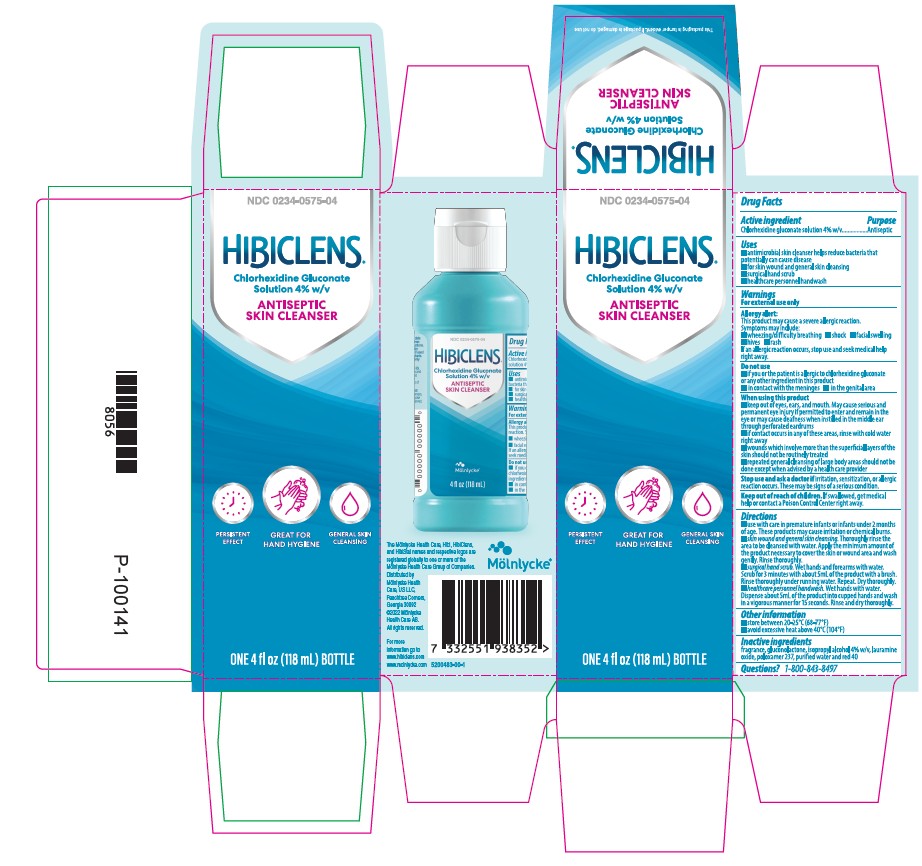
NDC 0234-0575-04
Hibiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
4oz (118mL)

NDC 0234-0575-91
Hibiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
1 gal (3.79L)

NDC 0234-0575-32
Hibiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
32oz (946mL)

NDC 0234-0575-16
Hibiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
16oz (473 mL)

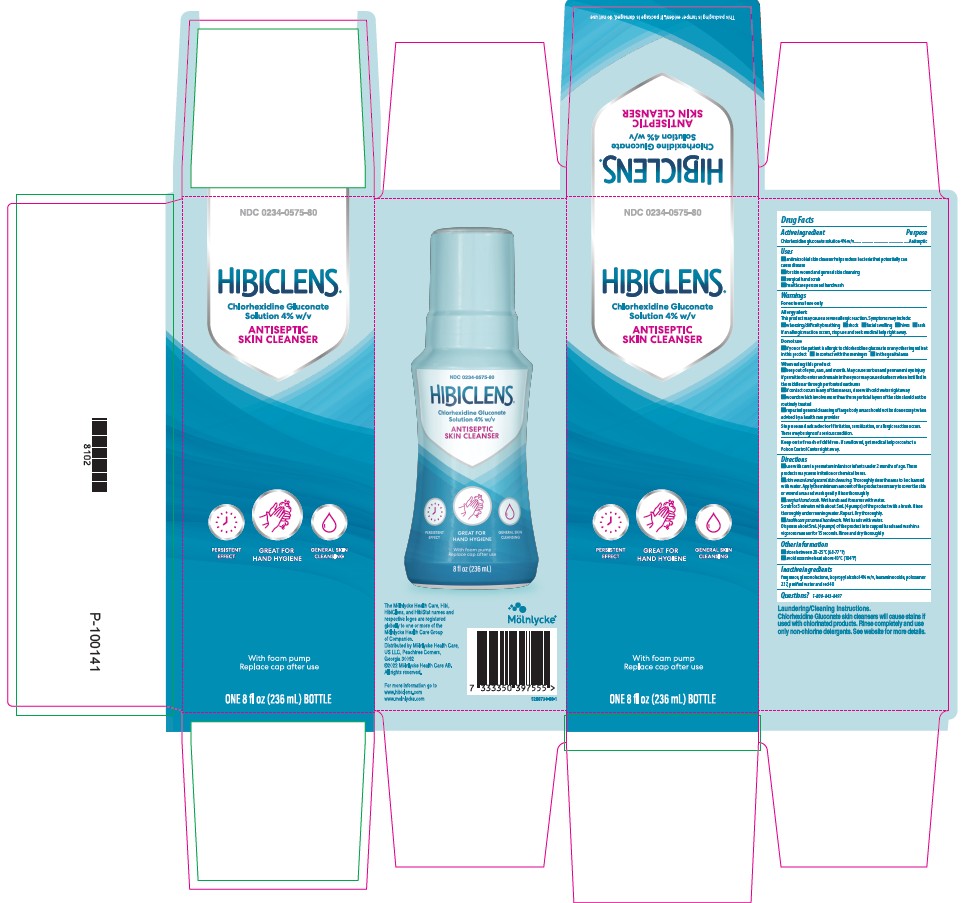
NDC 0234-0575-80
Hibiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
with foam pump Replace cap after use
8oz (236mL)

NDC 0234-0575-08
HIbiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
8oz (236mL)
NDC 0234-0575-41
Hibiclens
Chlorhexidine gluconate solution 4.0% w/v
Antiseptic Skin Cleanser
Persistent Effect
Great for Hand Hygiene
General Skin Cleansing
Helps reduce bacteria that potentially can cause disease
with foam pump Replace cap after use
4fl oz (118mL)

| HIBICLENS
chlorhexidine gluconate 4% solution |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Xttrium Laboratories, Inc. (007470579) |
| Registrant - Xttrium Laboratories, Inc. (007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Xttrium Laboratories, Inc. | 007470579 | manufacture(0116-0575) | |
More about Hibiclens (chlorhexidine topical)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (7)
- Latest FDA alerts (3)
- Side effects
- Dosage information
- During pregnancy
- Drug class: antiseptic and germicides
- Breastfeeding
- En español
Patient resources
Professional resources
Other brands
Peridex, Betasept, ChloraPrep One-Step, Paroex, ... +6 more