Fungizyl AL: Package Insert / Prescribing Info
Package insert / product label
Generic name: dimethyl sulfoxide and miconazole
Dosage form: topical liquid
Medically reviewed by Drugs.com. Last updated on Mar 4, 2025.
On This Page
Fungizyl AL Description
Fungizyl AL™ is a topical antifungal formulation that combines Dimethyl Sulfoxide (DMSO) and Miconazole Nitrate with a mixture of
emollients and essential oils. The formulation is designed to provide effective antifungal activity while also soothing and moisturizing the skin.
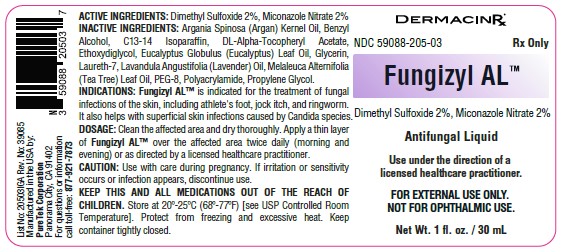
Fungizyl AL™ contains 20 mg of Dimethyl Sulfoxide and 20 mg of Miconazole Nitrate per gram in an anhydrous vehicle with Argania
Spinosa (Argan) Kernel Oil, Benzyl Alcohol, C13-14 Isoparaffin, DL-Alpha-Tocopheryl Acetate, Ethoxydiglycol, Eucalyptus Globulus (Eucalyptus) Leaf Oil, Glycerin, Laureth-7, Lavandula Angustifolia (Lavender) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, PEG-8,
Polyacrylamide and Propylene Glycol.
Indications and Usage for Fungizyl AL
Fungizyl AL™ is indicated for the treatment of fungal infections of the skin, including athlete's foot, jock itch, and ringworm. It also helps with superficial skin infections caused by Candida species. Dimethyl Sulfoxide (DMSO) enhances the penetration of Miconazole Nitrate through the skin, improving antifungal effectiveness.
Fungizyl AL - Clinical Pharmacology
Miconazole Nitrate: An antifungal agent that inhibits the biosynthesis of ergosterol, a key component of fungal cell membranes, resulting in increased cell permeability and leakage of cellular contents.
Dimethyl Sulfoxide (DMSO): A penetration enhancer that increases the absorption of Miconazole Nitrate through the skin, allowing for deeper antifungal action.
PHARMACOKINETICS:
When applied topically, Miconazole Nitrate exhibits minimal systemic absorption. Most of the drug remains on the skin surface and provides localized antifungal activity.
Contraindications
This product is contraindicated in patients with known hypersensitivity to any of its ingredients.
Warnings
For external use only. Not for ophthalmic use.
NURSING MOTHERS:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this drug is administered to a nursing mother.
Adverse Reactions/Side Effects
The most common side effects reported during the use of Miconazole-containing products are local irritation, burning, stinging, redness, or swelling at the application site.
Fungizyl AL Dosage and Administration
• Clean the affected area and dry thoroughly. Apply a thin layer of Fungizyl AL™ over the affected area twice daily (morning and evening) or as directed by a licensed healthcare practitioner.
• Continue treatment for at least 2 weeks, even if symptoms improve, to reduce the likelihood of recurrence.
• If there is no improvement after 4 weeks of treatment, discontinue use and consult a licensed healthcare practitioner.
How is Fungizyl AL supplied
Fungizyl AL™ is supplied in a 1 fl. oz. / 30 mL glass bottle with a screw cap fitted with a brush applicator (NDC 59088-205-03).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Store at 20º-25ºC (68º-77ºF) [see USP Controlled Room Temperature]. Protect from freezing and excessive heat. Keep container tightly closed.
| FUNGIZYL AL
dimethyl sulfoxide 2% and miconazole 2% liquid liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Puretek Corporation (785961046) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Puretek Corporation | 785961046 | manufacture(59088-205) | |