Fluticasone and Vilanterol Inhalation Powder: Package Insert / Prescribing Info
Package insert / product label
Dosage form: inhalation powder
Drug class: Bronchodilator combinations
Medically reviewed by Drugs.com. Last updated on Dec 5, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder, for oral inhalation use
Initial U.S. Approval: 2013
Indications and Usage for Fluticasone and Vilanterol Inhalation Powder
Fluticasone Furoate/Vilanterol ELLIPTA is a combination of fluticasone furoate, a corticosteroid, and vilanterol, a long‑acting beta2-adrenergic agonist (LABA), indicated for:
- •
- the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). (1.1)
- •
- the maintenance treatment of asthma in patients aged 5 years and older. (1.2)
Limitations of Use: Not indicated for relief of acute bronchospasm. (1.3, 5.2)
Fluticasone and Vilanterol Inhalation Powder Dosage and Administration
- •
- For oral inhalation only. (2.3)
- •
- Maintenance treatment of COPD: 1 actuation of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg once daily administered by oral inhalation. (2.1)
- •
- Maintenance treatment of asthma in adult patients aged 18 years and older: 1 actuation of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg or Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg once daily administered by oral inhalation. (2.2)
- •
- Maintenance treatment of asthma in pediatric patients aged 12 to 17 years: 1 actuation of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg once daily administered by oral inhalation. (2.2)
- •
- Maintenance treatment of asthma in pediatric patients aged 5 to 11 years: 1 actuation of fluticasone furoate/vilanterol ELLIPTA 50/25 mcg once daily administered by oral inhalation. (2.2)
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- •
- LABA monotherapy increases the risk of serious asthma-related events. (5.1)
- •
- Do not initiate in acutely deteriorating COPD or asthma. Do not use to treat acute symptoms. (5.2)
- •
- Do not use in combination with additional therapy containing a LABA because of risk of overdose. (5.3)
- •
- Candida albicans infection of the mouth and pharynx may occur. Monitor patients periodically. Advise the patient to rinse his/her mouth with water without swallowing after inhalation to help reduce the risk. (5.4)
- •
- Increased risk of pneumonia in patients with COPD. Monitor patients for signs and symptoms of pneumonia. (5.5)
- •
- Potential worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infections; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.6)
- •
- Risk of impaired adrenal function when transferring from systemic corticosteroids. Wean patients slowly from systemic corticosteroids if transferring to Fluticasone Furoate/Vilanterol ELLIPTA. (5.7)
- •
- Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue Fluticasone Furoate/Vilanterol ELLIPTA slowly. (5.8)
- •
- If paradoxical bronchospasm occurs, discontinue Fluticasone Furoate/Vilanterol ELLIPTA and institute alternative therapy. (5.10)
- •
- Use with caution in patients with cardiovascular disorders because of beta-adrenergic stimulation. (5.12)
- •
- Assess for decrease in bone mineral density (BMD) initially and periodically thereafter. (5.13)
- •
- Monitor growth of pediatric patients (5.14)
- •
- Glaucoma and cataracts may occur with long-term use of Inhaled Corticosteroid (ICS). Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Furoate/Vilanterol ELLIPTA long term. (5.15)
- •
- Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. (5.16)
- •
- Increased blood glucose levels have been reported. Also, be alert to hypokalemia. (5.17)
Adverse Reactions/Side Effects
- •
- COPD: Most common adverse reactions (incidence ≥3%) are nasopharyngitis, upper respiratory tract infection, headache, oral candidiasis, back pain, pneumonia, bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, hypertension, influenza, pharyngitis, and pyrexia. (6.1)
- •
- Asthma: Most common adverse reactions (incidence ≥2%) are nasopharyngitis, oral candidiasis, headache, influenza, upper respiratory tract infection, bronchitis, sinusitis, oropharyngeal pain, dysphonia, and cough. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Prasco Laboratories at 1-866-525-0688 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Strong cytochrome P450 3A4 inhibitors (e.g., ketoconazole): Use with caution. May cause systemic corticosteroid and cardiovascular effects. (7.1)
- •
- Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of vilanterol on cardiovascular system. (7.2)
- •
- Beta-blockers: Use with caution. May block bronchodilatory effects of beta-agonists and produce severe bronchospasm. (7.3)
- •
- Diuretics: Use with caution. Electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. (7.4)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2024
Full Prescribing Information
1. Indications and Usage for Fluticasone and Vilanterol Inhalation Powder
1.1 Maintenance Treatment of Chronic Obstructive Pulmonary Disease
Fluticasone Furoate/Vilanterol ELLIPTA is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
2. Fluticasone and Vilanterol Inhalation Powder Dosage and Administration
2.1 Recommended Dosage for Maintenance Treatment of Chronic Obstructive Pulmonary Disease
The recommended dosage of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (containing fluticasone furoate 100 mcg and vilanterol 25 mcg) is 1 actuation once daily by oral inhalation.
If shortness of breath occurs in the period between doses, an inhaled, short-acting beta2-agonist (rescue medicine, e.g., albuterol) should be used for immediate relief.
2.2 Recommended Dosage for Maintenance Treatment of Asthma
Adult Patients Aged 18 Years and Older
The recommended dosage of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (containing fluticasone furoate 100 mcg and vilanterol 25 mcg) is 1 actuation once daily by oral inhalation or Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg (containing fluticasone furoate 200 mcg and vilanterol 25 mcg) is 1 actuation once daily by oral inhalation.
- •
- When choosing the starting dosage strength of Fluticasone Furoate/Vilanterol ELLIPTA, consider the patients’ disease severity, their previous asthma therapy, including the inhaled corticosteroid (ICS) dosage, as well as the patients’ current control of asthma symptoms and risk of future exacerbation.
- •
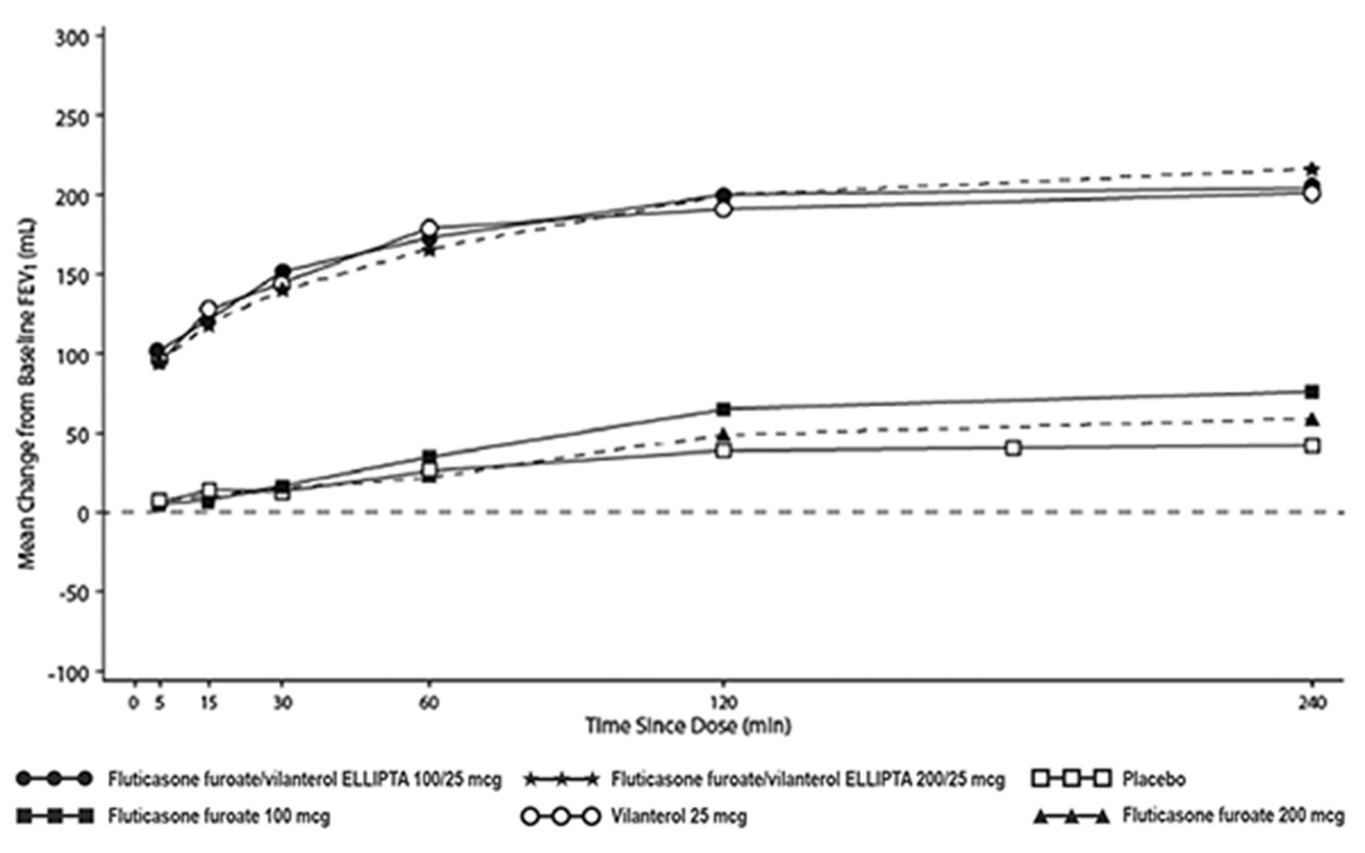
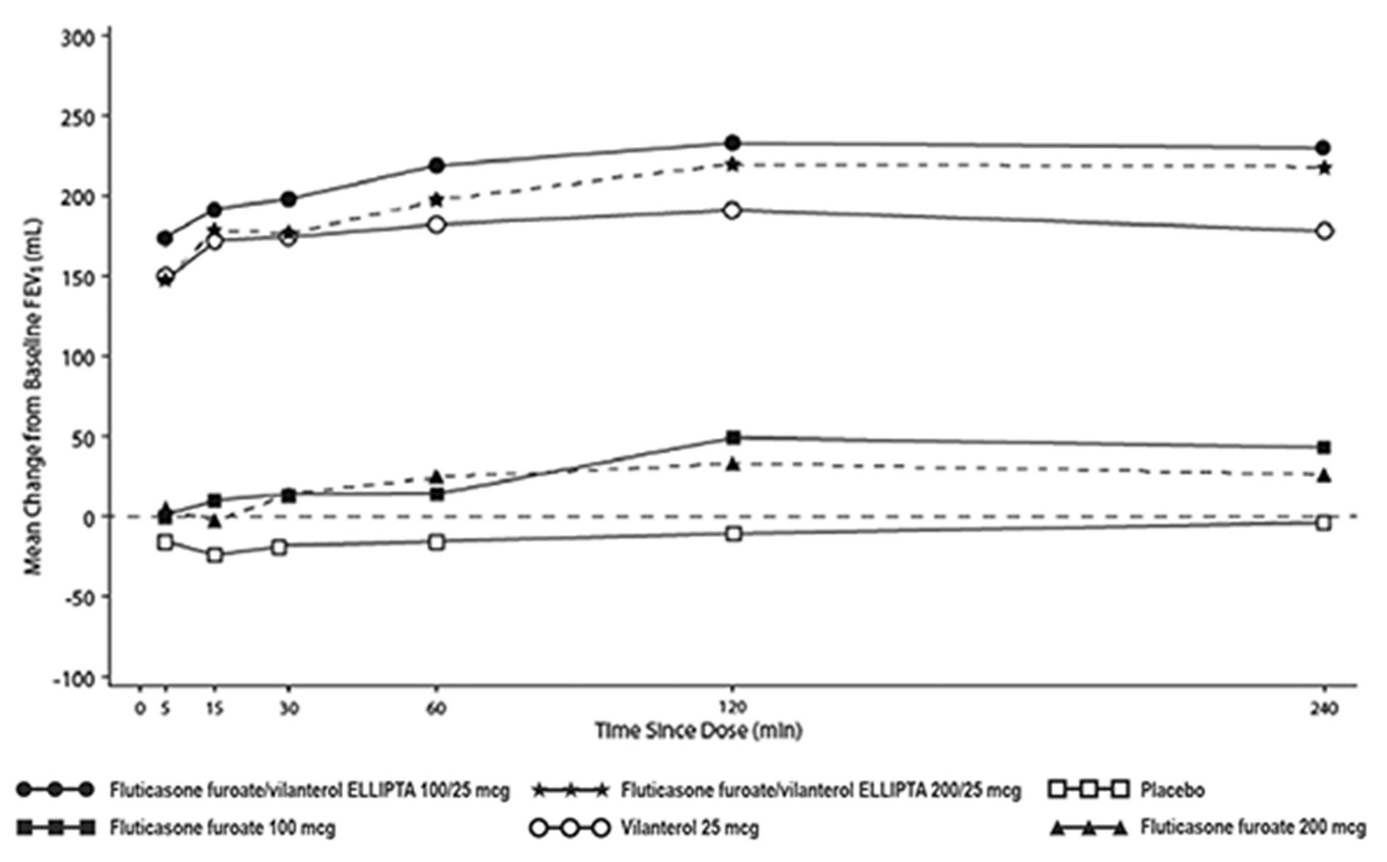
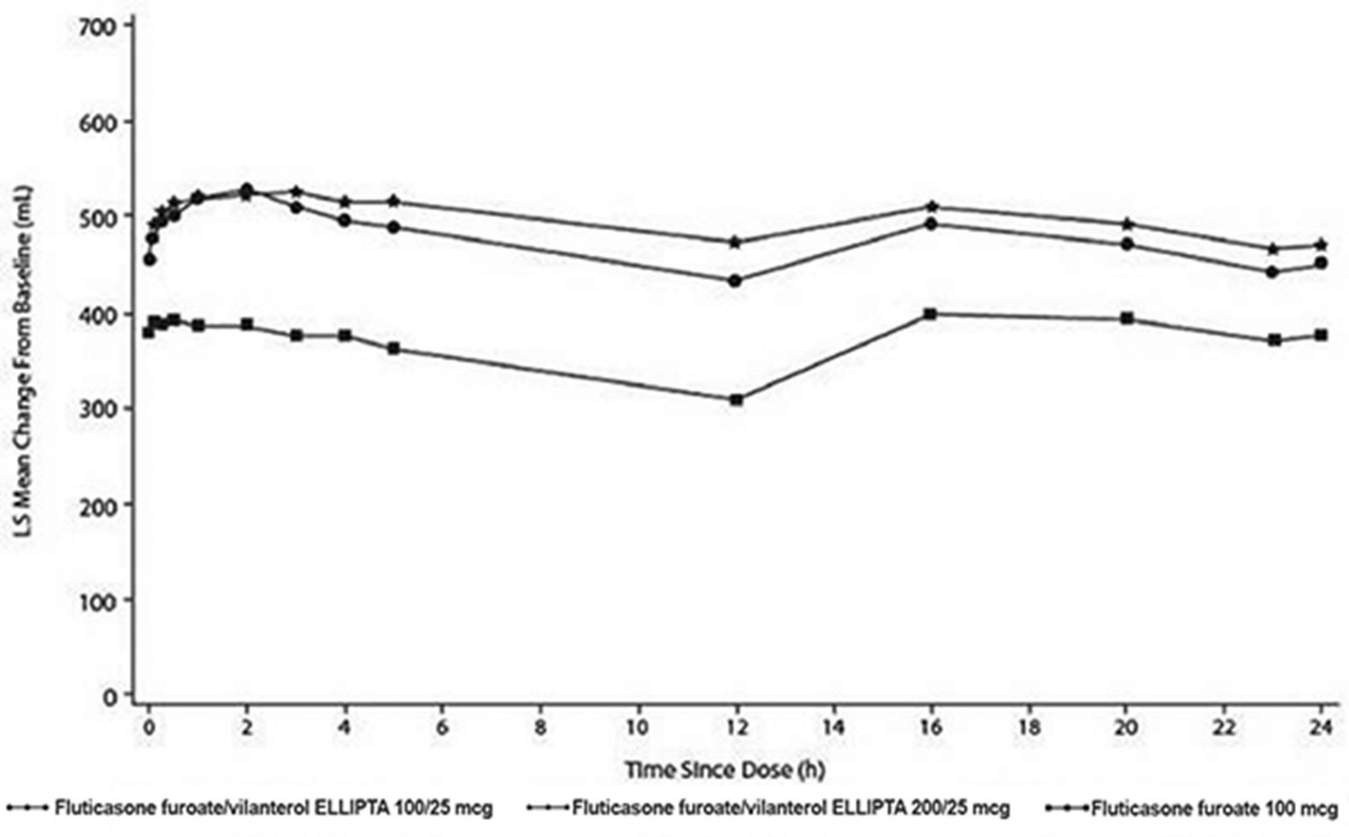
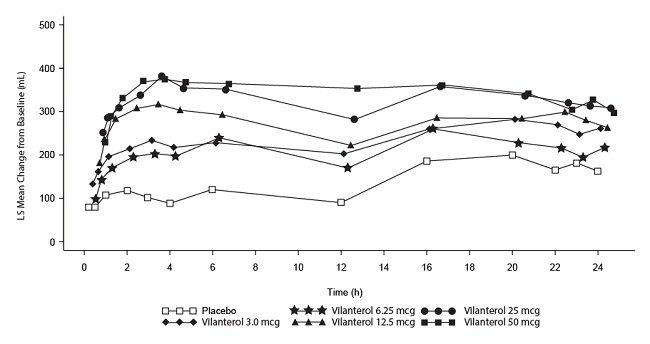
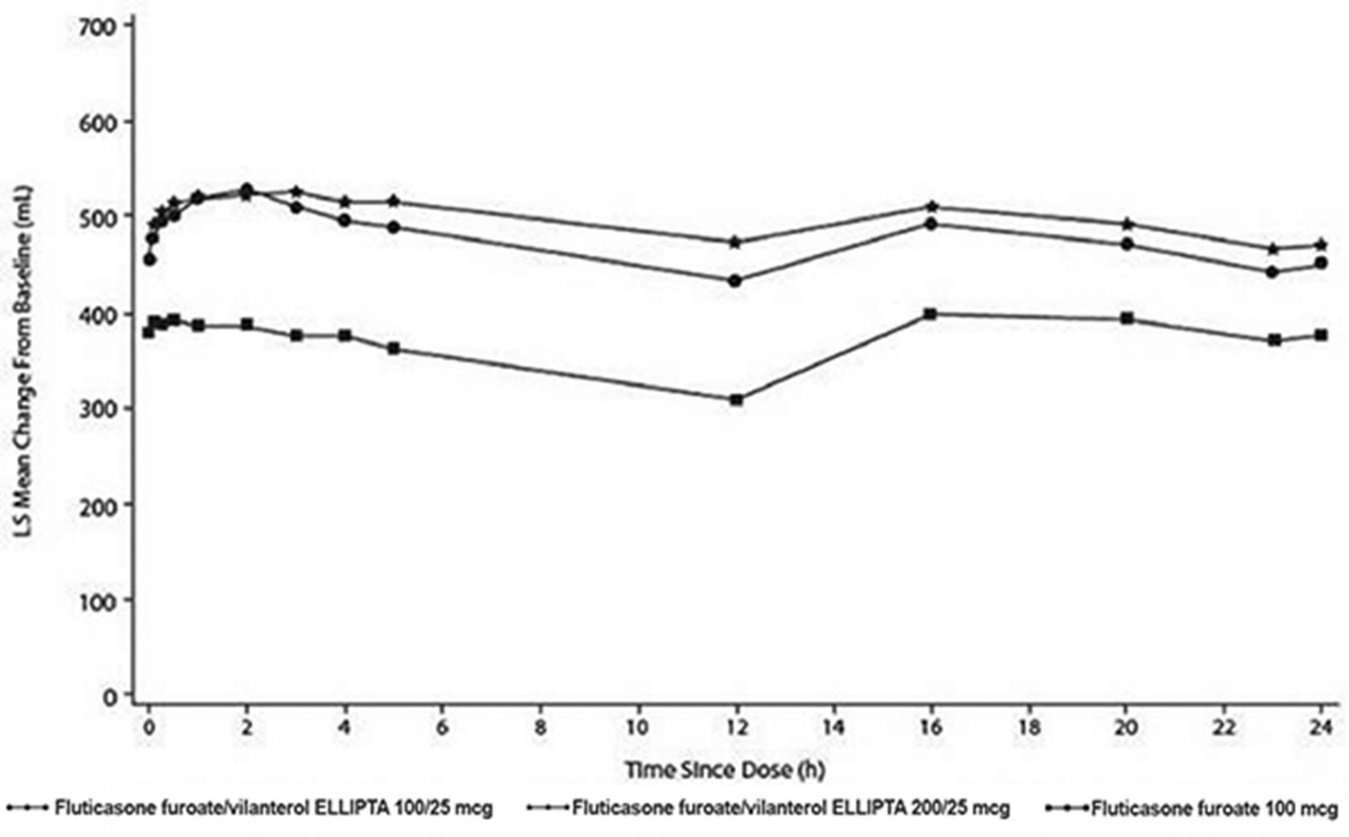
- The median time to onset, defined as a 100-mL increase from baseline in mean forced expiratory volume in 1 second (FEV1), was approximately 15 minutes after beginning treatment. Individual patients will experience a variable time to onset and degree of symptom relief.
- •
- For patients who do not respond adequately to Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg once daily, increasing the dose to Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg once daily may provide additional improvement in asthma control. For patients who do not respond adequately to Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg once daily, re-evaluate and consider other therapeutic regimens and additional therapeutic options.
- •
- The maximum recommended dosage is 1 inhalation of Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg once daily.
- •
- If asthma symptoms arise in the period between doses, an inhaled, short-acting beta2-agonist (rescue medicine, e.g., albuterol) should be used for immediate relief.
Pediatric Patients Aged 12 to 17 Years
The recommended dosage of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (containing fluticasone furoate 100 mcg and vilanterol 25 mcg) is 1 actuation once daily by oral inhalation [see Warnings and Precautions (5.14)].
Pediatric Patients Aged 5 to 11 Years
The recommended dosage of Fluticasone Furoate/Vilanterol ELLIPTA 50/25 mcg (containing fluticasone furoate 50 mcg and vilanterol 25 mcg) is 1 actuation once daily by oral inhalation [see Warnings and Precautions (5.14)].
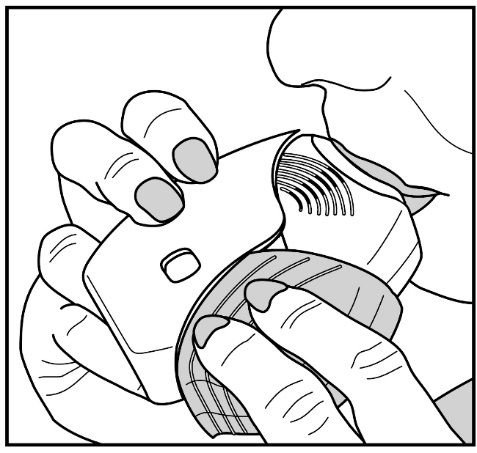
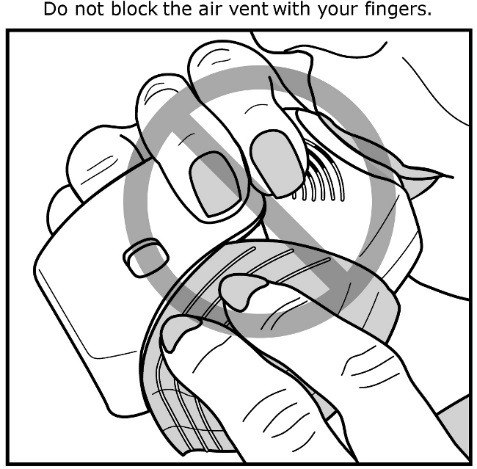
2.3 Administration Information
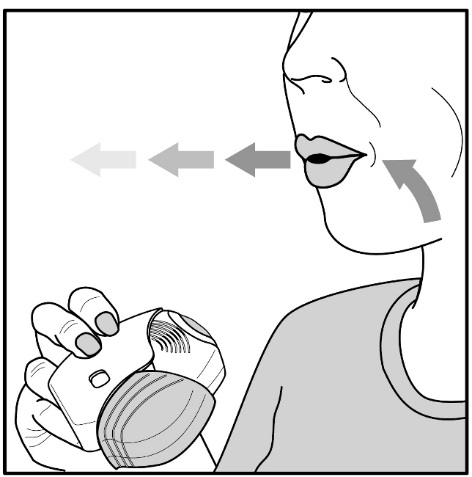
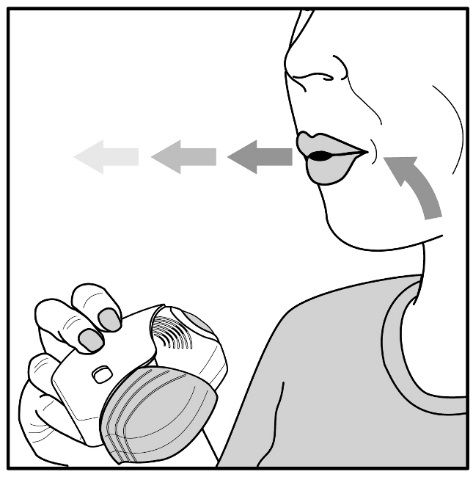
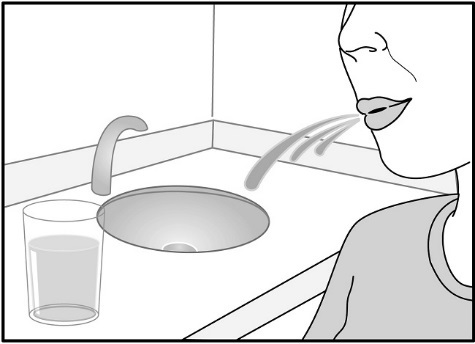
- •
- After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis [see Warnings and Precautions (5.4)].
- •
- Fluticasone Furoate/Vilanterol ELLIPTA should be used at the same time every day. Do not use Fluticasone Furoate/Vilanterol ELLIPTA more than 1 time every 24 hours.
- •
- More frequent administration or a greater number of inhalations (more than 1 inhalation daily) of the prescribed strength of Fluticasone Furoate/Vilanterol ELLIPTA is not recommended as some patients are more likely to experience adverse effects with higher doses.
3. Dosage Forms and Strengths
Inhalation powder:
- •
- 50 mcg fluticasone furoate and 25 mcg vilanterol (50/25 mcg) per actuation
- •
- 100 mcg fluticasone furoate and 25 mcg vilanterol (100/25 mcg) per actuation
- •
- 200 mcg fluticasone furoate and 25 mcg vilanterol (200/25 mcg) per actuation
4. Contraindications
Fluticasone Furoate/Vilanterol ELLIPTA is contraindicated in the following conditions:
- •
- Primary treatment of status asthmaticus or other acute episodes of COPD or asthma where intensive measures are required [see Warnings and Precautions (5.2)].
- •
- Severe hypersensitivity to milk proteins or demonstrated hypersensitivity to fluticasone furoate, vilanterol, or any of the excipients [see Warnings and Precautions (5.11), Description (11)].
5. Warnings and Precautions
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
Use of Long-acting Beta2-adrenergic Agonist (LABA) as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death [see Salmeterol Multicenter Asthma Research Trial (SMART)]. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma‑related events (hospitalizations, intubations, death) compared with ICS alone (see Serious Asthma-Related Events with Inhaled Corticosteroid/Long-Acting Beta2-Adrenergic Agonists).
Serious Asthma-Related Events with Inhaled Corticosteroid/Long-Acting Beta2-Adrenergic Agonists
Four (4) large, 26-week, randomized, double-blind, active-controlled clinical safety trials were conducted to evaluate the risk of serious asthma-related events when LABA were used in fixed‑dose combination with ICS compared with ICS alone in patients with asthma. Three (3) trials included adult and pediatric patients aged 12 years and older: 1 trial compared budesonide/formoterol with budesonide, 1 trial compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder, and 1 trial compared mometasone furoate/formoterol with mometasone furoate. The fourth trial included pediatric patients aged 4 to 11 years and compared fluticasone propionate/salmeterol inhalation powder with fluticasone propionate inhalation powder. The primary safety endpoint for all 4 trials was serious asthma-related events (hospitalizations, intubations, death). A blinded adjudication committee determined whether events were asthma related.
The 3 adult and pediatric trials were designed to rule out a risk margin of 2.0, and the pediatric trial was designed to rule out a risk margin of 2.7. Each individual trial met its pre-specified objective and demonstrated non-inferiority of ICS/LABA to ICS alone. A meta-analysis of the 3 adult and pediatric trials did not show a significant increase in risk of a serious asthma-related event with ICS/LABA fixed-dose combination compared with ICS alone (Table 1). These trials were not designed to rule out all risk for serious asthma-related events with ICS/LABA compared with ICS.
| ICS = Inhaled Corticosteroid, LABA = Long-acting Beta2-adrenergic Agonist. | |||
| a Randomized patients who had taken at least 1 dose of study drug. Planned treatment used for analysis. | |||
| b Estimated using a Cox proportional hazards model for time to first event with baseline hazards stratified by each of the 3 trials. | |||
| c Number of patients with event that occurred within 6 months after the first use of study drug or 7 days after the last date of study drug, whichever date was later. Patients can have one or more events, but only the first event was counted for analysis. A single, blinded, independent adjudication committee determined whether events were asthma related. | |||
|
ICS/LABA (n = 17,537)a |
ICS (n = 17,552)a |
ICS/LABA vs. ICS Hazard Ratio (95% CI)b |
|
|
Serious asthma-related eventc |
116 |
105 |
1.10 (0.85, 1.44) |
|
Asthma-related death |
2 |
0 | |
|
Asthma-related intubation (endotracheal) |
1 |
2 | |
|
Asthma-related hospitalization (≥24-hour stay) |
115 |
105 | |
The pediatric safety trial included 6,208 pediatric patients aged 4 to 11 years who received ICS/LABA (fluticasone propionate/salmeterol inhalation powder) or ICS (fluticasone propionate inhalation powder). In this trial, 27/3,107 (0.9%) patients randomized to ICS/LABA and 21/3,101 (0.7%) patients randomized to ICS experienced a serious asthma-related event. There were no asthma-related deaths or intubations. ICS/LABA did not show a significantly increased risk of a serious asthma-related event compared with ICS based on the pre-specified risk margin (2.7), with an estimated hazard ratio of time to first event of 1.29 (95% CI: 0.73, 2.27).
Salmeterol Multicenter Asthma Research Trial (SMART)
A 28-week, placebo-controlled, U.S. trial that compared the safety of salmeterol with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; relative risk: 4.37 [95% CI: 1.25, 15.34]). Use of background ICS was not required in SMART. The increased risk of asthma-related death is considered a class effect of LABA monotherapy.
5.2 Deterioration of Disease and Acute Episodes
Fluticasone Furoate/Vilanterol ELLIPTA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD or asthma. Fluticasone Furoate/Vilanterol ELLIPTA has not been studied in patients with acutely deteriorating COPD or asthma. The initiation of Fluticasone Furoate/Vilanterol ELLIPTA in this setting is not appropriate.
In COPD, if Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg no longer controls symptoms of bronchoconstriction; the patient’s inhaled, short-acting, beta2-agonist becomes less effective; or the patient needs more short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, re-evaluate the patient and the COPD treatment regimen at once. For COPD, the daily dose of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg should not be increased.
Increasing use of inhaled, short-acting beta2-agonists is a marker of deteriorating asthma. In this situation, the patient requires immediate reevaluation with reassessment of the treatment regimen, giving special consideration to the need for additional therapeutic options. Patients should not use more than 1 inhalation once daily of Fluticasone Furoate/Vilanterol ELLIPTA.
Fluticasone Furoate/Vilanterol ELLIPTA should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. Fluticasone Furoate/Vilanterol ELLIPTA has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
When beginning treatment with Fluticasone Furoate/Vilanterol ELLIPTA, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs and to use them only for symptomatic relief of acute respiratory symptoms. When prescribing Fluticasone Furoate/Vilanterol ELLIPTA, the healthcare provider should also prescribe an inhaled, short‑acting beta2-agonist and instruct the patient on how it should be used.
5.3 Risk Associated with Excessive Use of Long-Acting Beta2-Agonists, including Fluticasone Furoate/Vilanterol ELLIPTA
Fluticasone Furoate/Vilanterol ELLIPTA should not be used more often than recommended, at higher doses than recommended [see Dosage and Administration (2)], or in conjunction with other therapies containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using Fluticasone Furoate/Vilanterol ELLIPTA should not use another therapy containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
5.4 Oropharyngeal Candidiasis
Fluticasone Furoate/Vilanterol ELLIPTA contains fluticasone furoate, an ICS. Localized infections of the mouth and pharynx with Candida albicans have occurred in patients treated with orally inhaled drug products containing fluticasone furoate. Monitor patients periodically. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with Fluticasone Furoate/Vilanterol ELLIPTA continues. In some cases, therapy with Fluticasone Furoate/Vilanterol ELLIPTA may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following administration of Fluticasone Furoate/Vilanterol ELLIPTA to help reduce the risk of oropharyngeal candidiasis.
5.5 Pneumonia
An increase in the incidence of pneumonia has been observed in patients with COPD receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in clinical trials. There was also an increased incidence of pneumonias resulting in hospitalization. In some incidences these pneumonia events were fatal. Healthcare providers should remain vigilant for the possible development of pneumonia in patients with COPD as clinical features of pneumonia and exacerbations frequently overlap.
In replicate 12-month trials in 3,255 patients with moderate to severe COPD who had experienced a COPD exacerbation in the previous year, there was a higher incidence of pneumonia reported in patients receiving fluticasone furoate/vilanterol 50/25 mcg: 6% (48 of 820 patients); fluticasone furoate/vilanterol ELLIPTA 100/25 mcg: 6% (51 of 806 patients); or fluticasone furoate/vilanterol ELLIPTA 200/25 mcg: 7% (55 of 811 patients) than in patients receiving vilanterol 25 mcg: 3% (27 of 818 patients). There was no fatal pneumonia in patients receiving vilanterol or fluticasone furoate/vilanterol 50/25 mcg. There was fatal pneumonia in 1 patient receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg and in 7 patients receiving fluticasone furoate/vilanterol ELLIPTA 200/25 mcg (<1% for each treatment group).
In a mortality trial with a median treatment duration of 1.5 years in 16,568 patients with moderate COPD and cardiovascular disease, the annualized incidence rate of pneumonia was 3.4 per 100 patient-years for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 3.2 for placebo, 3.3 for fluticasone furoate 100 mcg, and 2.3 for vilanterol 25 mcg. Adjudicated, on-treatment deaths due to pneumonia occurred in 13 patients receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 9 patients receiving placebo, 10 patients receiving fluticasone furoate 100 mcg, and 6 patients receiving vilanterol 25 mcg (<0.2 per 100 patient-years for each treatment group).
5.6 Immunosuppression and Risk of Infections
Patients who are on drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chickenpox and measles can have a more serious or even fatal course in susceptible patients using corticosteroids. In such patients who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective Prescribing Information for VZIG and IG.) If chickenpox develops, treatment with antiviral agents may be considered.
ICS should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.7 Transferring Patients from Systemic Corticosteroid Therapy
Hypothalamic-Pituitary-Adrenal Suppression/Adrenal Insufficiency
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients during and after transfer from systemic corticosteroids to less systemically available ICS. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although Fluticasone Furoate/Vilanterol ELLIPTA may control COPD or asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress, a severe COPD exacerbation, or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their healthcare practitioner for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress, a severe COPD exacerbation, or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to Fluticasone Furoate/Vilanterol ELLIPTA. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with Fluticasone Furoate/Vilanterol ELLIPTA. Lung function (FEV1 or peak expiratory flow), beta-agonist use, and COPD or asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Unmasking of Allergic Conditions Previously Suppressed by Systemic Corticosteroids
Transfer of patients from systemic corticosteroid therapy to Fluticasone Furoate/Vilanterol ELLIPTA may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
Corticosteroid Withdrawal Symptoms
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
5.8 Hypercorticism and Adrenal Suppression
Inhaled fluticasone furoate is absorbed into the circulation and can be systemically active. Effects of fluticasone furoate on the HPA axis are not observed with the therapeutic doses of fluticasone furoate in Fluticasone Furoate/Vilanterol ELLIPTA. However, exceeding the recommended dosage or coadministration with a strong cytochrome P450 3A4 (CYP3A4) inhibitor may result in HPA dysfunction [see Warnings and Precautions (5.9), Drug Interactions (7.1)].
Because of the possibility of significant systemic absorption of ICS in sensitive patients, patients treated with Fluticasone Furoate/Vilanterol ELLIPTA should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, reduce the dose of Fluticasone Furoate/Vilanterol ELLIPTA slowly, consistent with accepted procedures for reducing systemic corticosteroids, and consider other treatments for management of COPD or asthma symptoms.
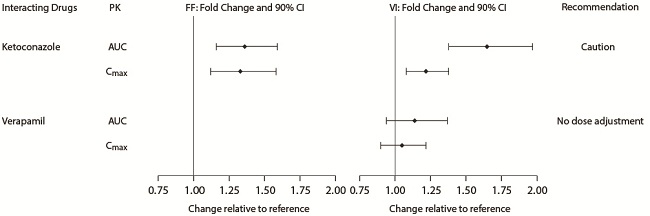
5.9 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the coadministration of Fluticasone Furoate/Vilanterol ELLIPTA with ketoconazole and other known strong CYP3A4 inhibitors (including, but not limited to, ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) because increased systemic corticosteroid and increased cardiovascular adverse effects may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
5.10 Paradoxical Bronchospasm
Fluticasone Furoate/Vilanterol ELLIPTA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with Fluticasone Furoate/Vilanterol ELLIPTA, it should be treated immediately with an inhaled, short-acting bronchodilator; Fluticasone Furoate/Vilanterol ELLIPTA should be discontinued immediately; and alternative therapy should be instituted [see Adverse Reactions (6.3)].
5.11 Hypersensitivity Reactions, including Anaphylaxis
Hypersensitivity reactions such as anaphylaxis, angioedema, rash, and urticaria may occur after administration of Fluticasone Furoate/Vilanterol ELLIPTA. Discontinue Fluticasone Furoate/Vilanterol ELLIPTA if such reactions occur. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder medications containing lactose; therefore, patients with severe milk protein allergy should not use Fluticasone Furoate/Vilanterol ELLIPTA [see Contraindications (4), Adverse Reactions (6.3)].
5.12 Cardiovascular Effects
Fluticasone Furoate/Vilanterol ELLIPTA, like other drugs containing beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles [see Adverse Reactions (6.3)]. If such effects occur, Fluticasone Furoate/Vilanterol ELLIPTA may need to be discontinued. In addition, beta‑agonists have been reported to produce electrocardiographic changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown [see Clinical Pharmacology (12.2)]. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
In healthy subjects, large doses of inhaled fluticasone furoate/vilanterol (4 times the recommended dose of vilanterol, representing a 12- or 10-fold higher systemic exposure than seen in patients with COPD or asthma, respectively) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias. Therefore, Fluticasone Furoate/Vilanterol ELLIPTA, like other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
In a mortality trial with a median treatment duration of 1.5 years in 16,568 patients with moderate COPD and cardiovascular disease, the annualized incidence rate of adjudicated cardiovascular events (composite of myocardial infarction, stroke, unstable angina, transient ischemic attack, or on-treatment death due to cardiovascular events) was 2.5 per 100 patient‑years for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 2.7 for placebo, 2.4 for fluticasone furoate 100 mcg, and 2.6 for vilanterol 25 mcg. Adjudicated, on-treatment deaths due to cardiovascular events occurred in 82 patients receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 86 patients receiving placebo, 80 patients receiving fluticasone furoate 100 mcg, and 90 patients receiving vilanterol 25 mcg (annualized incidence rate ranged from 1.2 to 1.3 per 100 patient-years for the treatment groups).
5.13 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing ICS. The clinical significance of small changes in BMD with regard to long‑term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care. Since patients with COPD often have multiple risk factors for reduced BMD, assessment of BMD is recommended prior to initiating Fluticasone Furoate/Vilanterol ELLIPTA and periodically thereafter. If significant reductions in BMD are seen and Fluticasone Furoate/Vilanterol ELLIPTA is still considered medically important for that patient’s COPD therapy, use of therapy to treat or prevent osteoporosis should be strongly considered.
In replicate 12-month trials in 3,255 patients with moderate to severe COPD, bone fractures were reported by 2% of patients receiving the fluticasone furoate/vilanterol combination (50/25 mcg: 2% [14 of 820 patients]; 100/25 mcg: 2% [19 of 806 patients]; or 200/25 mcg: 2% [14 of 811 patients]) compared with <1% of patients receiving vilanterol 25 mcg alone (8 of 818 patients).
Similar findings were seen in a mortality trial with a median treatment duration of 1.5 years in 16,568 patients with moderate COPD and cardiovascular disease.
5.14 Effect on Growth
Orally inhaled corticosteroids, including fluticasone furoate, a component in Fluticasone Furoate/Vilanterol ELLIPTA may cause a reduction in growth velocity when administered to pediatric patients. The safety and effectiveness of Fluticasone Furoate/Vilanterol ELLIPTA have not been established in pediatric patients less than 5 years of age. Monitor the growth of pediatric patients receiving Fluticasone Furoate/Vilanterol ELLIPTA routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including Fluticasone Furoate/Vilanterol ELLIPTA, titrate each patient’s dose to the lowest dosage that effectively controls his/her symptoms [see Dosage and Administration (2.3), Use in Specific Populations (8.4)].
5.15 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients with COPD or asthma following the long-term administration of ICS, including fluticasone furoate, a component in Fluticasone Furoate/Vilanterol ELLIPTA. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use Fluticasone Furoate/Vilanterol ELLIPTA long term.
5.16 Risk of Using Sympathomimetic Amines in Certain Coexisting Conditions
Fluticasone Furoate/Vilanterol ELLIPTA, like all therapies containing sympathomimetic amines, should be used with caution in patients with convulsive disorders, thyrotoxicosis, or diabetes mellitus and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2‑adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.17 Hyperglycemia and Hypokalemia
There have been reports of increases in blood glucose levels with fluticasone furoate/vilanterol ELLIPTA. This should be considered in patients with a history of, or with risk factors for, diabetes mellitus [see Adverse Reactions (6.3)].
Beta-adrenergic agonist therapies may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. In clinical trials evaluating fluticasone furoate/vilanterol ELLIPTA in patients with COPD or asthma, there was no evidence of a treatment effect on serum potassium.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in labeling:
- •
- Serious Asthma-Related Events – Hospitalizations, Intubations, Death [see Warnings and Precautions (5.1)]
- •
- Oropharyngeal Candidiasis [see Warnings and Precautions (5.4)]
- •
- Pneumonia [see Warnings and Precautions (5.5)]
- •
- Immunosuppression and Risk of Infections [see Warnings and Precautions (5.6)]
- •
- Hypercorticism and Adrenal Suppression [see Warnings and Precautions (5.8)]
- •
- Paradoxical Bronchospasm [see Warnings and Precautions (5.10)]
- •
- Cardiovascular Effects [see Warnings and Precautions (5.12)]
- •
- Reduction in Bone Mineral Density [see Warnings and Precautions (5.13)]
- •
- Growth Effects [see Warnings and Precautions (5.14)]
- •
- Glaucoma and Cataracts [see Warnings and Precautions (5.15)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
The safety data described below are based on two 6-month and two 12-month trials and one long-term mortality trial. In these studies, 5,356 patients with COPD received at least 1 dose of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg. Adverse reactions observed in other studies of fluticasone furoate/vilanterol ELLIPTA in COPD patients were similar to those observed in these 5 trials.
6-Month Trials
The incidence of adverse reactions associated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in Table 2 is based on 2 placebo-controlled, 6-month clinical trials (Trials 1 and 2; n = 1,224 and n = 1,030, respectively). Of the 2,254 patients, 70% were male and 84% were White. They had a mean age of 62 years and an average smoking history of 44 pack years, with 54% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 48% (range: 14% to 87%), the mean postbronchodilator FEV1/forced vital capacity (FVC) ratio was 47% (range: 17% to 88%), and the mean percent reversibility was 14% (range: -41% to 152%).
Patients received 1 inhalation once daily of the following: fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, fluticasone furoate 100 mcg, fluticasone furoate 200 mcg, vilanterol 25 mcg, or placebo.
| a Includes oral candidiasis, oropharyngeal candidiasis, candidiasis, and fungal oropharyngitis. | ||||
|
Adverse Reaction |
Fluticasone Furoate/
100/25 mcg (n = 410) % |
Vilanterol 25 mcg (n = 408) % |
Fluticasone Furoate 100 mcg (n = 410) % |
Placebo (n = 412) % |
|
Infections and infestations | ||||
|
Nasopharyngitis |
9 |
10 |
8 |
8 |
|
Upper respiratory tract infection |
7 |
5 |
4 |
3 |
|
Oropharyngeal candidiasisa |
5 |
2 |
3 |
2 |
|
Nervous system disorders | ||||
|
Headache |
7 |
9 |
7 |
5 |
12-Month Trials
Long-term safety data are based on two 12-month trials (Trials 3 and 4; n = 1,633 and n = 1,622, respectively). Trials 3 and 4 included 3,255 patients, of which 57% were male and 85% were White. They had a mean age of 64 years and an average smoking history of 46 pack years, with 44% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 45% (range: 12% to 91%), and the mean postbronchodilator FEV1/FVC ratio was 46% (range: 17% to 81%), indicating that the patient population had moderate to very severely impaired airflow obstruction. Patients received 1 inhalation once daily of the following: fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, or vilanterol 25 mcg. In addition to the reactions shown in Table 2, adverse reactions occurring in ≥3% of the patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 806) for 12 months included back pain, pneumonia [see Warnings and Precautions (5.5)], bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, influenza, pharyngitis, and pyrexia.
Mortality Trial
Safety data are available from a mortality trial in patients with moderate COPD (moderate airflow limitation [≥50% and ≤70% predicted FEV1]) who either had a history of, or were at risk of, cardiovascular disease and were treated for up to 4 years (median treatment duration of 1.5 years). The trial included 16,568 patients, 4,140 of whom received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg. In addition to the events in COPD trials shown in Table 2, adverse reactions occurring in ≥3% of the patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg and more common than placebo included pneumonia, back pain, hypertension, and influenza.
6.2 Clinical Trials Experience in Asthma
The safety data described below are based on trials that evaluated fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in 1,757 patients and fluticasone furoate/vilanterol ELLIPTA 200/25 mcg in 745 patients. While patients aged 12 to 17 years were included in these trials, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg is not approved for use in this age group [see Dosage and Administration (2.2)].
One additional 24-week trial enrolled 902 pediatric patients with asthma. In this trial, fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was studied in 117 patients aged 12 to 17 years and fluticasone furoate/vilanterol ELLIPTA 50/25 mcg was studied in 337 patients aged 5 to 11 years.
Adult Patients
The safety of fluticasone furoate/vilanterol ELLIPTA for the maintenance treatment of asthma in adult patients was based on the data from Trials 8, 9, 10, 11, and 12 [see Clinical Studies (14.2)]. Trial 8 was a 12-week trial that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg in patients with asthma compared with fluticasone furoate 100 mcg and placebo. The incidence of adverse reactions associated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg is shown in Table 3.
| a Includes oral candidiasis and oropharyngeal candidiasis. | |||
|
Adverse Reaction |
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (n = 201) % |
Fluticasone Furoate 100 mcg (n = 205) % |
Placebo (n = 203) % |
|
Infections and infestations | |||
|
Nasopharyngitis |
10 |
7 |
7 |
|
Oral candidiasisa |
2 |
2 |
0 |
|
Nervous system disorders | |||
|
Headache |
5 |
4 |
4 |
|
Respiratory, thoracic, and mediastinal disorders | |||
|
Oropharyngeal pain |
2 |
2 |
1 |
|
Dysphonia |
2 |
1 |
0 |
Trial 9 was a 12-week trial that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, and fluticasone furoate 100 mcg in patients with asthma. This trial did not have a placebo arm. The incidence of adverse reactions associated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg and fluticasone furoate/vilanterol ELLIPTA 200/25 mcg is shown in Table 4.
|
Adverse Reaction |
Fluticasone Furoate/Vilanterol ELLIPTA 200/25 mcg (n = 346) % |
Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg (n = 346) % |
Fluticasone Furoate 100 mcg (n = 347) % |
|
Nervous system disorders | |||
|
Headache |
8 |
8 |
9 |
|
Infections and infestations | |||
|
Nasopharyngitis |
7 |
6 |
7 |
|
Influenza |
3 |
3 |
1 |
|
Upper respiratory tract infection |
2 |
2 |
3 |
|
Sinusitis |
2 |
1 |
<1 |
|
Bronchitis |
2 |
<1 |
2 |
|
Respiratory, thoracic, and mediastinal disorders | |||
|
Oropharyngeal pain |
2 |
2 |
1 |
|
Cough |
1 |
2 |
1 |
24-Week Trial
Trial 10 was a 24-week trial that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 200/25 mcg once daily, fluticasone furoate 200 mcg once daily, and fluticasone propionate 500 mcg twice daily in patients with asthma. This trial did not have a placebo arm. In addition to the reactions shown in Tables 3 and 4, adverse reactions occurring in ≥2% of patients treated with fluticasone furoate/vilanterol ELLIPTA 200/25 mcg included viral respiratory tract infection, pharyngitis, pyrexia, and arthralgia.
12-Month Trial
Long-term safety data are based on a 12-month trial that evaluated the safety of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily (n = 201), fluticasone furoate/vilanterol ELLIPTA 200/25 mcg once daily (n = 202), and fluticasone propionate 500 mcg twice daily (n = 100) in patients with asthma (Trial 11). In addition to the reactions shown in Tables 3 and 4, adverse reactions occurring in ≥2% of the patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg or fluticasone furoate/vilanterol ELLIPTA 200/25 mcg for 12 months included pyrexia, back pain, extrasystoles, upper abdominal pain, respiratory tract infection, allergic rhinitis, pharyngitis, rhinitis, arthralgia, supraventricular extrasystoles, ventricular extrasystoles, acute sinusitis, and pneumonia.
Adult and Pediatric Patients Aged 12 to 17 Years Exacerbation Trial
Trial 12 included both adult and pediatric patients 12 years of age and older. Although this trial did not support efficacy of fluticasone furoate/vilanterol ELLIPTA for maintenance treatment of asthma in pediatric patients 12 to 17 years of age, it was used to evaluate safety in both adult and pediatric patients 12 to 17 years of age. Patients received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 1,009) or fluticasone furoate 100 mcg (n = 1,010). Patients participating in this trial had a history of 1 or more asthma exacerbations that required treatment with oral/systemic corticosteroids or emergency department visit or in-patient hospitalization for the treatment of asthma in the year prior to trial entry. Asthma-related hospitalizations occurred in 10 patients (1%) treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with 7 patients (0.7%) treated with fluticasone furoate 100 mcg. Among patients aged 12 to 17 years, asthma-related hospitalizations occurred in 4 patients (2.6%) treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 151) compared with 0 patients treated with fluticasone furoate 100 mcg (n = 130). There were no asthma-related deaths or asthma-related intubations observed in this trial.
Pediatric Patients Aged 5 to 17 Years
The safety of fluticasone furoate/vilanterol ELLIPTA for the maintenance treatment of asthma in pediatric patients 5 years and older was based on the data from Trial 14, a 24-week clinical trial that enrolled 902 patients with asthma aged 5 to 17 years (aged 5 to 11 years [n = 673]; aged 12 to 17 years [n = 229]). Pediatric patients aged 12 to 17 years were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 117) or fluticasone furoate 100 mcg (n = 112). Pediatric patients aged 5 to 11 years were randomized to fluticasone furoate/vilanterol ELLIPTA 50/25 mcg (n = 337) or fluticasone furoate 50 mcg (n = 336) [see Clinical Studies (14.2)]. Adverse reactions reported in ≥3% of pediatric patients treated with fluticasone furoate/vilanterol ELLIPTA is shown in Table 5.
| a The dose of fluticasone furoate/vilanterol ELLIPTA was 100/25 mcg once daily for pediatric patients aged 12 to 17 years and 50/25 mcg once daily for pediatric patients aged 5 to 11 years. b The dose of fluticasone furoate was 100 mcg once daily for pediatric patients aged 12 to 17 years and 50 mcg once daily for pediatric patients aged 5 to 11 years. |
||||||||||
|
Adverse Reaction |
Fluticasone Furoate/Vilanterol ELLIPTAa (n = 454) % |
Fluticasone Furoateb (n = 448) % |
||||||||
|
Infections and infestations | ||||||||||
|
Nasopharyngitis |
10 |
8 |
||||||||
|
Upper respiratory tract infection |
7 |
6 |
||||||||
|
Rhinitis |
3 |
1 |
||||||||
|
Viral upper respiratory tract infection |
3 |
<1 |
||||||||
|
Respiratory, thoracic, and mediastinal disorders | ||||||||||
|
Rhinitis allergic |
4 |
1 |
||||||||
|
Nervous system disorders | ||||||||||
|
Headache |
3 |
2 |
||||||||
6.3 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during post approval use of fluticasone furoate/vilanterol ELLIPTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone furoate/vilanterol ELLIPTA or a combination of these factors.
Cardiac Disorders
Palpitations, tachycardia.
Immune System Disorders
Hypersensitivity reactions, including anaphylaxis, angioedema, rash, and urticaria.
Metabolism and Nutrition Disorders
Hyperglycemia.
Musculoskeletal and Connective Tissue Disorders
Muscle spasms.
Nervous System Disorders
Tremor.
Psychiatric Disorders
Nervousness.
Respiratory, Thoracic, and Mediastinal Disorders
Paradoxical bronchospasm.
7. Drug Interactions
7.1 Inhibitors of Cytochrome P450 3A4
Fluticasone furoate and vilanterol are both substrates of CYP3A4. Concomitant administration of the strong CYP3A4 inhibitor ketoconazole increases the systemic exposure to fluticasone furoate and vilanterol. Caution should be exercised when considering the coadministration of Fluticasone Furoate/Vilanterol ELLIPTA with ketoconazole and other known strong CYP3A4 inhibitors [see Warnings and Precautions (5.9), Clinical Pharmacology (12.3)].
7.2 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, and QTc Prolonging Drugs
Vilanterol, like other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval or within 2 weeks of discontinuation of such agents, because the effect of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval have an increased risk of ventricular arrhythmias.
7.3 Beta-Adrenergic Receptor Blocking Agents
Beta-blockers not only block the pulmonary effect of beta-agonists, such as vilanterol, but may also produce severe bronchospasm in patients with COPD or asthma. Therefore, patients with COPD or asthma should not normally be treated with beta-blockers. However, under certain circumstances, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents for these patients; cardioselective beta-blockers could be considered, although they should be administered with caution.
7.4 Non–Potassium-Sparing Diuretics
The electrocardiographic changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with non–potassium-sparing diuretics.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are insufficient data on the use of fluticasone furoate/vilanterol ELLIPTA or its individual components, fluticasone furoate and vilanterol, in pregnant women to inform a drug-associated risk. (See Clinical Considerations.) In an animal reproduction study, fluticasone furoate and vilanterol administered by inhalation alone or in combination to pregnant rats during the period of organogenesis produced no fetal structural abnormalities. The highest fluticasone furoate and vilanterol doses in this study were approximately 5 and 40 times the maximum recommended human daily inhalation doses (MRHDID) of 200 and 25 mcg, respectively. (See Data.)
The estimated risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryofetal Risk: In women with poorly or moderately controlled asthma, there is an increased risk of several perinatal outcomes such as pre-eclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. Pregnant women should be closely monitored, and medication adjusted as necessary to maintain optimal control of asthma.
Labor or Delivery: Fluticasone Furoate/Vilanterol ELLIPTA should be used during late gestation and labor only if the potential benefit justifies the potential for risks related to beta-agonists interfering with uterine contractility.
Data
Animal Data: Fluticasone Furoate and Vilanterol: In an embryofetal developmental study, pregnant rats received fluticasone furoate and vilanterol during the period of organogenesis at doses up to approximately 5 and 40 times the MRHDID of 200 and 25 mcg, respectively, alone or in combination (on a mcg/m2 basis at inhalation doses up to approximately 95 mcg/kg/day). No evidence of structural abnormalities was observed.
Fluticasone Furoate: In 2 separate embryofetal developmental studies, pregnant rats and rabbits received fluticasone furoate during the period of organogenesis at doses up to approximately 4 and 1 times, respectively, the MRHDID of 200 mcg (on a mcg/m2 basis at maternal inhalation doses up to 91 and 8 mcg/kg/day, respectively). No evidence of structural abnormalities in fetuses was observed in either species. In a perinatal and postnatal developmental study in rats, dams received fluticasone furoate during late gestation and lactation periods at doses up to approximately 1 time the MRHDID of 200 mcg (on a mcg/m2 basis at maternal inhalation doses up to 27 mcg/kg/day). No evidence of effects on offspring development was observed.
Vilanterol: In 2 separate embryofetal developmental studies, pregnant rats and rabbits received vilanterol during the period of organogenesis at doses up to approximately 13,000 and 1,000 times, respectively, the MRHDID (on a mcg/m2 basis at maternal inhalation doses up to 33,700 mcg/kg/day in rats and on an AUC basis at maternal inhaled doses up to 5,740 mcg/kg/day in rabbits). No evidence of structural abnormalities was observed at any dose in rats or in rabbits up to approximately 160 times the MRHDID (on an AUC basis at maternal doses up to 591 mcg/kg/day). However, fetal skeletal variations were observed in rabbits at approximately 1,000 times the MRHDID (on an AUC basis at maternal inhaled or subcutaneous doses of 5,740 or 300 mcg/kg/day, respectively). The skeletal variations included decreased or absent ossification in cervical vertebral centrum and metacarpals. In a perinatal and postnatal developmental study in rats, dams received vilanterol during late gestation and the lactation periods at doses up to approximately 3,900 times the MRHDID (on a mcg/m2 basis at maternal oral doses up to 10,000 mcg/kg/day). No evidence of effects in offspring development was observed.
8.2 Lactation
Risk Summary
There is no information available on the presence of fluticasone furoate or vilanterol in human milk, the effects on the breastfed child, or the effects on milk production. Low concentrations of other ICS have been detected in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Fluticasone Furoate/Vilanterol ELLIPTA and any potential adverse effects on the breastfed child from fluticasone furoate or vilanterol or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Fluticasone Furoate/Vilanterol ELLIPTA for the maintenance treatment of asthma in pediatric patients 5 years of age and older have been established. This indication is based on Trial 14, an adequate and well-controlled trial in pediatric patients aged 5 to 17 years [see Adverse Reactions (6.2), Clinical Studies (14.2)]. The recommended dosage for pediatric patients is different than the adult dosage [see Dosage and Administration (2.2)].
The safety and efficacy of Fluticasone Furoate/Vilanterol ELLIPTA in pediatric patients aged younger than 5 years have not been established.
In Trial 12, an exacerbation trial [see Clinical Studies (14.2)], pediatric patients aged 12 to 17 years (n = 281) were treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 151) or treated with fluticasone furoate 100 mcg (n = 130). Among these patients, 10% of patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reported an asthma exacerbation compared with 7% for patients treated with fluticasone furoate 100 mcg. Asthma-related hospitalizations occurred in 4 patients (2.6%) treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with 0 patients treated with fluticasone furoate 100 mcg.
Effects on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. A reduction of growth velocity in pediatric patients may occur as a result of poorly controlled asthma or from use of corticosteroids, including ICS. The effects of long-term treatment of pediatric patients with ICS, including fluticasone furoate, on final adult height are not known.
Controlled clinical trials have shown that ICS may cause a reduction in growth in children. In these trials, the mean reduction in growth velocity was approximately 1 cm/year (range: 0.3 to 1.8 cm/year) and appears to be related to dose and duration of exposure. This effect has been observed in the absence of laboratory evidence of HPA axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in children than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for “catch-up” growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied. The growth of pediatric patients receiving orally inhaled corticosteroids, including Fluticasone Furoate/Vilanterol ELLIPTA, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including Fluticasone Furoate/Vilanterol ELLIPTA, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
A randomized, double-blind, parallel-group, multicenter, 1-year, placebo-controlled trial evaluated the effect of once-daily treatment with orally inhaled fluticasone furoate 50 mcg on growth velocity assessed by stadiometry. The patients were 457 prepubertal children (girls aged 5 to <8 years and boys aged 5 to <9 years). Mean growth velocity over the 52-week treatment period was lower in the patients receiving orally inhaled fluticasone furoate (5.905 cm/year) compared with placebo (6.065 cm/year). The mean reduction in growth velocity was 0.16 cm/year (95% CI: -0.14, 0.46) [see Warnings and Precautions (5.14)].
8.5 Geriatric Use
Based on available data, no adjustment of the dosage of Fluticasone Furoate/Vilanterol ELLIPTA in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
Clinical trials of fluticasone furoate/vilanterol ELLIPTA for COPD included 4,820 subjects aged 65 years and older and 1,118 subjects aged 75 years and older. Clinical trials of fluticasone furoate/vilanterol ELLIPTA for asthma included 854 subjects aged 65 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients.
8.6 Hepatic Impairment
Fluticasone furoate systemic exposure increased by up to 3‑fold in adult patients with hepatic impairment compared with healthy subjects. Hepatic impairment had no effect on vilanterol systemic exposure. Use Fluticasone Furoate/Vilanterol ELLIPTA with caution in patients with moderate or severe hepatic impairment. Monitor patients for corticosteroid-related side effects. The effect of hepatic impairment on fluticasone furoate and vilanterol systemic exposure in patients aged younger than 18 years has not been evaluated [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
There were no significant increases in either fluticasone furoate or vilanterol exposure in patients with severe renal impairment (CrCl <30 mL/min) compared with healthy subjects. No dosage adjustment is required in patients with renal impairment [see Clinical Pharmacology (12.3)].
10. Overdosage
Fluticasone Furoate/Vilanterol ELLIPTA contains both fluticasone furoate and vilanterol; therefore, the risks associated with overdosage for the individual components described below apply to Fluticasone Furoate/Vilanterol ELLIPTA. Treatment of overdosage consists of discontinuation of Fluticasone Furoate/Vilanterol ELLIPTA together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta‑receptor blocker may be considered, bearing in mind that such medicine can produce bronchospasm. Cardiac monitoring is recommended in cases of overdosage.
Fluticasone Furoate
Because of low systemic bioavailability (15.2%) and an absence of acute drug-related systemic findings in clinical trials, overdosage of fluticasone furoate is unlikely to require any treatment other than observation. If used at excessive doses for prolonged periods, systemic effects such as hypercorticism may occur [see Warnings and Precautions (5.8)].
Vilanterol
The expected signs and symptoms with overdosage of vilanterol are those of excessive beta‑adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of beta-adrenergic stimulation (e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis). As with all inhaled sympathomimetic medicines, cardiac arrest and even death may be associated with an overdose of vilanterol.
11. Fluticasone and Vilanterol Inhalation Powder Description
Fluticasone Furoate/Vilanterol ELLIPTA is an inhalation powder drug product for delivery of a combination of fluticasone furoate (an ICS) and vilanterol (a LABA) to patients by oral inhalation.
Fluticasone furoate, a synthetic trifluorinated corticosteroid, has the chemical name (6α,11β,16α,17α)-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl 2-furancarboxylate and the following chemical structure:
Fluticasone furoate is a white powder with a molecular weight of 538.6, and the empirical formula is C27H29F3O6S. It is practically insoluble in water.
Vilanterol trifenatate has the chemical name triphenylacetic acid-4-{(1R)-2-[(6-{2-[2,6-dicholorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol (1:1) and the following chemical structure:
Vilanterol trifenatate is a white powder with a molecular weight of 774.8, and the empirical formula is C24H33Cl2NO5•C20H16O2. It is practically insoluble in water.
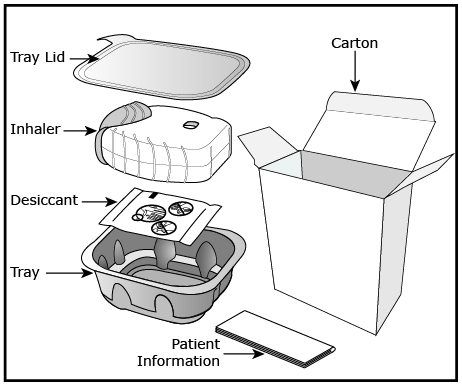
Fluticasone furoate/vilanterol ELLIPTA is a light grey and pale blue plastic inhaler containing 2 foil blister strips. Each blister on one strip contains a white powder blend of micronized fluticasone furoate (50, 100 or 200 mcg) and lactose monohydrate (12.5, 12.4 or 12.3 mg, respectively), and each blister on the other strip contains a white powder blend of micronized vilanterol trifenatate (40 mcg equivalent to 25 mcg of vilanterol), magnesium stearate (125 mcg), and lactose monohydrate (12.34 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within both blisters is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, fluticasone furoate/vilanterol ELLIPTA delivers 46, 92 or 184 mcg of fluticasone furoate and 22 mcg of vilanterol per dose when tested at a flow rate of 60 L/min for 4 seconds.
In adult patients with obstructive lung disease and severely compromised lung function (COPD with FEV1/FVC <70% and FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 66.5 L/min (range: 43.5 to 81.0 L/min).
In adult patients with severe asthma, mean peak inspiratory flow through the ELLIPTA inhaler was 96.6 L/min (range: 72.4 to 124.6 L/min). In pediatric patients with asthma aged 5 to 11 years, mean peak inspiratory flow through the ELLIPTA inhaler was 60.6 L/min (range: 36.3 to 82.5 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
12. Fluticasone and Vilanterol Inhalation Powder - Clinical Pharmacology
12.1 Mechanism of Action
Fluticasone Furoate/Vilanterol ELLIPTA
Fluticasone Furoate/Vilanterol ELLIPTA contains both fluticasone furoate and vilanterol. The mechanisms of action described below for the individual components apply to Fluticasone Furoate/Vilanterol ELLIPTA. These drugs represent 2 different classes of medications (an ICS and a LABA), each having different effects on clinical and physiological indices.
Fluticasone Furoate
Fluticasone furoate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone furoate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is approximately 29.9 times that of dexamethasone and 1.7 times that of fluticasone propionate. The clinical relevance of these findings is unknown.
The precise mechanism through which fluticasone furoate affects COPD and asthma symptoms is not known. Inflammation is an important component in the pathogenesis of COPD and asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. Specific effects of fluticasone furoate demonstrated in in vitro and in vivo models included activation of the glucocorticoid response element, inhibition of pro-inflammatory transcription factors such as NFkB, and inhibition of antigen-induced lung eosinophilia in sensitized rats. These anti-inflammatory actions of corticosteroids may contribute to their efficacy.
Vilanterol
Vilanterol is a LABA. In vitro tests have shown the functional selectivity of vilanterol was similar to salmeterol. The clinical relevance of this in vitro finding is unknown.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenergic agonist drugs, including vilanterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′,5′-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Healthy Subjects: QTc interval prolongation was studied in a double-blind, multiple-dose, placebo- and positive-controlled crossover study in 85 healthy volunteers. The maximum mean (95% upper confidence bound) difference in QTcF from placebo after baseline correction was 4.9 (7.5) milliseconds and 9.6 (12.2) milliseconds seen 30 minutes after dosing for fluticasone furoate/vilanterol 200/25 mcg and fluticasone furoate/vilanterol 800/100 mcg, respectively.
A dose-dependent increase in heart rate was also observed. The maximum mean (95% upper confidence bound) difference in heart rate from placebo after baseline-correction was 7.8 (9.4) beats/min and 17.1 (18.7) beats/min seen 10 minutes after dosing for fluticasone furoate/vilanterol 200/25 mcg and fluticasone furoate/vilanterol 800/100 mcg, respectively.
Hypothalamic-Pituitary-Adrenal Axis Effects
Healthy Subjects: Inhaled fluticasone furoate at repeat doses up to 400 mcg was not associated with statistically significant decreases in serum or urinary cortisol in healthy subjects. Decreases in serum and urine cortisol levels were observed at fluticasone furoate exposures several-fold higher than exposures observed at the therapeutic dose.
Patients with Chronic Obstructive Pulmonary Disease: In a trial with patients with COPD, treatment with fluticasone furoate (50, 100, or 200 mcg)/vilanterol 25 mcg, vilanterol 25 mcg, or fluticasone furoate (100 or 200 mcg) for 6 months did not affect 24-hour urinary cortisol excretion. A separate trial with patients with COPD demonstrated no effects on serum cortisol after 28 days of treatment with fluticasone furoate (50, 100, or 200 mcg)/vilanterol 25 mcg.
Patients with Asthma: A randomized, double-blind, parallel-group trial in 104 pediatric patients with asthma (aged 5 to 11 years) showed no difference between once-daily treatment with inhaled fluticasone furoate 50 mcg compared with placebo on serum cortisol weighted mean (0 to 24 hours) and serum cortisol AUC(0-24) following 6 weeks of treatment.
A randomized, double-blind, parallel-group trial in 185 patients with asthma aged 12 to 65 years showed no difference between once-daily treatment with fluticasone furoate/vilanterol 100/25 mcg or fluticasone furoate/vilanterol 200/25 mcg compared with placebo on serum cortisol weighted mean (0 to 24 hours), serum cortisol AUC(0-24), and 24-hour urinary cortisol after 6 weeks of treatment, whereas prednisolone 10 mg given once daily for 7 days resulted in significant cortisol suppression.
12.3 Pharmacokinetics
Linear pharmacokinetics was observed for fluticasone furoate (200 to 800 mcg) and vilanterol (25 to 100 mcg). On repeated once-daily inhalation administration, steady state of fluticasone furoate and vilanterol plasma concentrations was achieved after 6 days, and the accumulation was up to 2.6-fold for fluticasone furoate and 2.4-fold for vilanterol as compared with single dose.
Absorption
Fluticasone Furoate: Fluticasone furoate plasma levels may not predict therapeutic effect. Peak plasma concentrations are reached within 0.5 to 1 hour. Absolute bioavailability of fluticasone furoate when administered by inhalation was 15.2%, primarily due to absorption of the inhaled portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose is low (approximately 1.3%) due to extensive first-pass metabolism. Systemic exposure (AUC) in patients with COPD or asthma was 46% or 7% lower, respectively, than observed in healthy subjects.
Vilanterol: Vilanterol plasma levels may not predict therapeutic effect. Peak plasma concentrations are reached within 10 minutes following inhalation. Absolute bioavailability of vilanterol when administered by inhalation was 27.3%, primarily due to absorption of the inhaled portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose of vilanterol is low (<2%) due to extensive first-pass metabolism. Systemic exposure (AUC) in patients with COPD was 24% higher than observed in healthy subjects. Systemic exposure (AUC) in patients with asthma was 21% lower than observed in healthy subjects.
Distribution
Fluticasone Furoate: Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 661 L. Binding of fluticasone furoate to human plasma proteins was high (>99%).
Vilanterol: Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 165 L. Binding of vilanterol to human plasma proteins was on average 94%.
Elimination
Metabolism: Fluticasone Furoate: Fluticasone furoate is cleared from systemic circulation principally by hepatic metabolism via CYP3A4 to metabolites with significantly reduced corticosteroid activity. There was no in vivo evidence for cleavage of the furoate moiety resulting in the formation of fluticasone.
Vilanterol: Vilanterol is mainly metabolized, principally via CYP3A4, to a range of metabolites with significantly reduced β1- and β2-agonist activity.
Excretion: Fluticasone Furoate: Fluticasone furoate and its metabolites are eliminated primarily in the feces, accounting for approximately 101% and 90% of the orally and intravenously administered doses, respectively. Urinary excretion accounted for approximately 1% and 2% of the orally and intravenously administered doses, respectively. Following repeat-dose inhaled administration, the plasma elimination phase half-life averaged 24 hours.
Vilanterol: Following oral administration, vilanterol was eliminated mainly by metabolism followed by excretion of metabolites in urine and feces (approximately 70% and 30%, respectively, of the recovered radioactive dose). The plasma elimination half-life of vilanterol, as determined from inhalation administration of multiple doses of vilanterol 25 mcg, is 21.3 hours in patients with COPD and 16.0 hours in patients with asthma.
Specific Populations
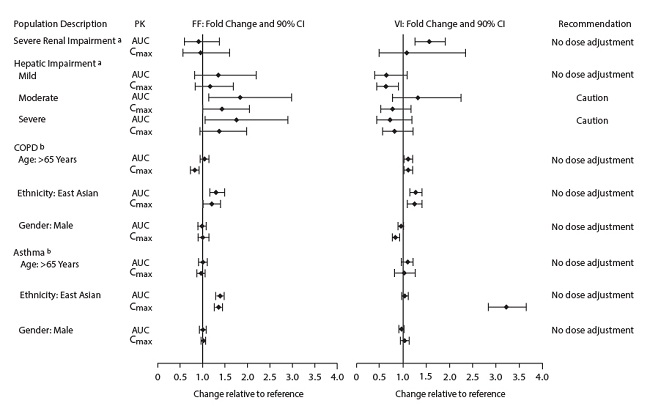
The effects of renal and hepatic impairment and other intrinsic factors on the pharmacokinetics of fluticasone furoate and vilanterol are shown in Figure 1.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination
a Severe renal impairment (CrCl <30 mL/min) compared with healthy subjects; mild (Child‑Pugh A), moderate (Child-Pugh B), and severe (Child-Pugh C) hepatic impairment compared with healthy subjects.
b For COPD and asthma, the following comparisons were made: age compared with ≤65 years, gender compared with female, and ethnicity compared with White.
Pediatric Patients: Fluticasone Furoate: A population pharmacokinetics analysis to assess impact of age on fluticasone furoate systemic exposure was conducted using combined data from clinical trials in pediatric patients aged 5 to 11 years (n = 306). There was no relevant effect of age on the apparent clearance of fluticasone furoate. The dose-adjusted fluticasone furoate systemic exposure at steady state in children aged 5 to 11 years following 50 mcg were comparable to that observed in adult and pediatric patients 12 years and older following dosing with fluticasone furoate 100 mcg monotherapy.
Vilanterol: A population pharmacokinetic analysis was conducted to characterize vilanterol pharmacokinetics using combined data from clinical trials in pediatric patients aged 5 to 11 years (n = 142). There was no relevant effect of age, weight, body mass index, sex, ethnicity, and race on vilanterol clearance. A cross-study comparison in pediatric patients with asthma showed that at steady-state, when combined with fluticasone furoate, vilanterol had similar AUC values but lower Cmax values compared to vilanterol administered alone. Vilanterol systemic exposure at steady state, in patients with asthma aged 5 to 11 years, was similar to those observed in adult and pediatric patients 12 years and older with asthma following repeat dosing of Fluticasone Furoate/Vilanterol ELLIPTA 100/25 mcg.
Racial or Ethnic Groups: Fluticasone Furoate: Systemic exposure [AUC(0-24)] to inhaled fluticasone furoate 200 mcg was 27% to 49% higher in healthy subjects of Japanese, Korean, and Chinese heritage compared with White subjects. Similar differences were observed for patients with COPD or asthma (Figure 1). However, there is no evidence that this higher exposure to fluticasone furoate results in clinically relevant effects on urinary cortisol excretion or on efficacy in these racial groups.
Vilanterol: There was no effect of race on the pharmacokinetics of vilanterol in patients with COPD. In patients with asthma, vilanterol Cmax is estimated to be higher (3-fold) and AUC(0-24) comparable for those patients from an Asian heritage compared with patients from a non-Asian heritage. However, the higher Cmax values are similar to those seen in healthy subjects.
Patients with Hepatic Impairment: Fluticasone Furoate: Following repeat dosing of fluticasone furoate/vilanterol 200/25 mcg (100/12.5 mcg in the severe impairment group) for 7 days, there was an increase of 34%, 83%, and 75% in fluticasone furoate systemic exposure (AUC) in patients with mild, moderate, and severe hepatic impairment, respectively, compared with healthy subjects (Figure 1).
In patients with moderate hepatic impairment receiving fluticasone furoate/vilanterol 200/25 mcg, mean serum cortisol (0 to 24 hours) was reduced by 34% (90% CI: 11%, 51%) compared with healthy subjects. In patients with severe hepatic impairment receiving fluticasone furoate/vilanterol 100/12.5 mcg, mean serum cortisol (0 to 24 hours) was increased by 14% (90% CI: -16%, 55%) compared with healthy subjects. Patients with moderate to severe hepatic disease should be closely monitored.
Vilanterol: Hepatic impairment had no effect on vilanterol systemic exposure [Cmax and AUC(0-24) on Day 7] following repeat-dose administration of fluticasone furoate/vilanterol 200/25 mcg (100/12.5 mcg in the severe impairment group) for 7 days (Figure 1).
There were no additional clinically relevant effects of the fluticasone furoate/vilanterol combinations on heart rate or serum potassium in patients with mild or moderate hepatic impairment (vilanterol 25 mcg combination) or with severe hepatic impairment (vilanterol 12.5 mcg combination) compared with healthy subjects.
Patients with Renal Impairment: Fluticasone furoate systemic exposure was not increased and vilanterol systemic exposure [AUC(0-24)] was 56% higher in patients with severe renal impairment compared with healthy subjects (Figure 1). There was no evidence of greater corticosteroid or beta-agonist class-related systemic effects (assessed by serum cortisol, heart rate, and serum potassium) in patients with severe renal impairment compared with healthy subjects.
Drug Interaction Studies
There were no clinically relevant differences in the pharmacokinetics or pharmacodynamics of either fluticasone furoate or vilanterol when administered in combination compared with administration alone. The potential for fluticasone furoate and vilanterol to inhibit or induce metabolic enzymes and transporter systems is negligible at low inhalation doses.
Inhibitors of Cytochrome P450 3A4: The exposure (AUC) of fluticasone furoate and vilanterol were 36% and 65% higher, respectively, when coadministered with ketoconazole 400 mg compared with placebo (Figure 2). The increase in fluticasone furoate exposure was associated with a 27% reduction in weighted mean serum cortisol (0 to 24 hours). The increase in vilanterol exposure was not associated with an increase in beta-agonist–related systemic effects on heart rate or blood potassium.
Figure 2. Impact of Coadministered Drugsa on the Pharmacokinetics (PK) of Fluticasone Furoate (FF) and Vilanterol (VI) Following Administration as Fluticasone Furoate/Vilanterol Combination or Vilanterol Coadministered with a Long-Acting Muscarinic Antagonist
a Compared with placebo group.
Inhibitors of P-glycoprotein: Fluticasone furoate and vilanterol are both substrates of P‑glycoprotein (P-gp). Coadministration of repeat-dose (240 mg once daily) verapamil (a moderate CYP3A4 inhibitor and a P-gp inhibitor) did not affect the vilanterol Cmax or AUC in healthy subjects (Figure 2). Drug interaction trials with a specific P-gp inhibitor and fluticasone furoate have not been conducted.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Fluticasone Furoate/Vilanterol ELLIPTA
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with fluticasone furoate/vilanterol ELLIPTA; however, studies are available for the individual components, fluticasone furoate and vilanterol, as described below.
Fluticasone Furoate
Fluticasone furoate produced no treatment-related increases in the incidence of tumors in 2-year inhalation studies in rats and mice at inhaled doses up to 9 and 19 mcg/kg/day, respectively (both approximately 0.5 times the MRHDID of 200 mcg on a mcg/m2 basis).
Fluticasone furoate did not induce gene mutation in bacteria or chromosomal damage in a mammalian cell mutation test in mouse lymphoma L5178Y cells in vitro. There was also no evidence of genotoxicity in the in vivo micronucleus test in rats.
No evidence of impairment of fertility was observed in male and female rats at inhaled fluticasone furoate doses up to 29 and 91 mcg/kg/day, respectively (approximately 3 and 8 times, respectively, the MRHDID of 200 mcg for adults on an AUC basis).
Vilanterol
In a 2-year carcinogenicity study in mice, vilanterol caused a statistically significant increase in ovarian tubulostromal adenomas in females at an inhaled dose of 29,500 mcg/kg/day (approximately 8,750 times the MRHDID on an AUC basis). No increase in tumors was seen at an inhaled dose of 615 mcg/kg/day (approximately 530 times the MRHDID on an AUC basis).
In a 2-year carcinogenicity study in rats, vilanterol caused statistically significant increases in mesovarian leiomyomas in females and shortening of the latency of pituitary tumors at inhaled doses greater than or equal to 84.4 mcg/kg/day (greater than or equal to approximately 45 times the MRHDID on an AUC basis). No tumors were seen at an inhalation dose of 10.5 mcg/kg/day (approximately 2 times the MRHDID on an AUC basis).
These tumor findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Vilanterol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vivo rat bone marrow micronucleus assay, in vivo rat unscheduled DNA synthesis (UDS) assay, and in vitro Syrian hamster embryo (SHE) cell assay. Vilanterol tested equivocal in the in vitro mouse lymphoma assay.
No evidence of impairment of fertility was observed in male and female rats at inhaled vilanterol doses up to 31,500 and 37,100 mcg/kg/day, respectively (both approximately 5,490 times the MRHDID based on AUC).
14. Clinical Studies
14.1 Chronic Obstructive Pulmonary Disease
Four trials evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA on lung function (Trial 1, NCT01053988 and Trial 2, NCT01054885) and exacerbations (Trial 3, NCT01009463 and Trial 4, NCT01017952).
Lung Function: Trials 1 and 2 were 24-week, randomized, double-blind, placebo-controlled trials designed to evaluate the efficacy of fluticasone furoate/vilanterol ELLIPTA on lung function in patients with COPD. In Trial 1, patients were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate 100 mcg, fluticasone furoate 200 mcg, vilanterol 25 mcg, and placebo. In Trial 2, patients were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, fluticasone furoate 100 mcg, vilanterol 25 mcg, and placebo. All treatments were administered as 1 inhalation once daily.
Of the 2,254 patients, 70% were male and 84% were White. They had a mean age of 62 years and an average smoking history of 44 pack years, with 54% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 48% (range: 14% to 87%), mean postbronchodilator FEV1/FVC ratio was 47% (range: 17% to 88%), and the mean percent reversibility was 14% (range: -41% to 152%).
The co-primary efficacy variables in both trials were weighted mean FEV1 (0 to 4 hours) postdose on Day 168 and change from baseline in trough FEV1 on Day 169 (the mean of the FEV1 values obtained 23 and 24 hours after the final dose on Day 168). The weighted mean comparison of the fluticasone furoate/vilanterol combination with fluticasone furoate was assessed to evaluate the contribution of vilanterol to fluticasone furoate/vilanterol ELLIPTA. The trough FEV1 comparison of the fluticasone furoate/vilanterol combination with vilanterol was assessed to evaluate the contribution of fluticasone furoate to fluticasone furoate/vilanterol ELLIPTA.
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg demonstrated a larger increase in the weighted mean FEV1 (0 to 4 hours) relative to placebo and fluticasone furoate 100 mcg at Day 168 (Table 6).
| FEV1 = Forced Expiratory Volume in 1 second. a At Day 168. b At Day 169. |
||||||
|
Treatment |
N |
Weighted Mean FEV1 (0-4 h)a (mL) |
Trough FEV1b (mL) |
|||
|
Difference from |
Difference from |
|||||
|
Placebo (95% CI) |
Fluticasone Furoate 100 mcg (95% CI) |
Fluticasone Furoate 200 mcg (95% CI) |
Placebo (95% CI) |
Vilanterol 25 mcg (95% CI) |
||
|
Trial 1 |
||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
204 |
214 (161, 266) |
168 (116, 220) |
–– |
144 (91, 197) |
45 (-8, 97) |
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
205 |
209 (157, 261) |
–– |
168 (117, 219) |
131 (80, 183) |
32 (-19, 83) |
|
Trial 2 |
||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
206 |
173 (123, 224) |
120 (70, 170) |
–– |
115 (60, 169) |
48 (-6, 102) |
Serial spirometric evaluations were performed predose and up to 4 hours after dosing. Results from Trial 1 at Day 1 and Day 168 are shown in Figure 3. Similar results were seen in Trial 2 (not shown).
Figure 3. Raw Mean Change from Baseline in Postdose Serial FEV1 (0-4 h) (mL) on Days 1 and 168
Day 1
Day 168
The second co-primary variable was change from baseline in trough FEV1 following the final treatment day. At Day 169, both Trials 1 and 2 demonstrated significant increases in trough FEV1 for all strengths of the fluticasone furoate/vilanterol combination compared with placebo (Table 7). The comparison of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg with vilanterol did not achieve statistical significance (Table 7).
Trials 1 and 2 evaluated FEV1 as a secondary endpoint. Peak FEV1 was defined as the maximum postdose FEV1 recorded within 4 hours after the first dose of trial medicine on Day 1 (measurements recorded at 5, 15, and 30 minutes and 1, 2, and 4 hours). In both trials, differences in mean change from baseline in peak FEV1 were observed for the groups receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo (152 and 139 mL, respectively). The median time to onset, defined as a 100-mL increase from baseline in FEV1, was 16 minutes in patients receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg.
Exacerbations: Trials 3 and 4 were randomized, double-blind, 52-week trials designed to evaluate the effect of fluticasone furoate/vilanterol ELLIPTA on the rate of moderate and severe COPD exacerbations. All patients were treated with fluticasone propionate/salmeterol 250/50 mcg twice daily during a 4-week run-in period prior to being randomly assigned to 1 of the following treatment groups: fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate/vilanterol 50/25 mcg, or vilanterol 25 mcg.
The primary efficacy variable in both trials was the annual rate of moderate/severe exacerbations. The comparison of the fluticasone furoate/vilanterol combination with vilanterol was assessed to evaluate the contribution of fluticasone furoate to fluticasone furoate/vilanterol ELLIPTA. In these 2 trials, exacerbations were defined as worsening of 2 or more major symptoms (dyspnea, sputum volume, and sputum purulence) or worsening of any 1 major symptom together with any 1 of the following minor symptoms: sore throat, colds (nasal discharge and/or nasal congestion), fever without other cause, and increased cough or wheeze for at least 2 consecutive days. COPD exacerbations were considered to be of moderate severity if treatment with systemic corticosteroids and/or antibiotics was required and were considered to be severe if hospitalization was required.
Trials 3 and 4 included 3,255 patients, of which 57% were male and 85% were White. They had a mean age of 64 years and an average smoking history of 46 pack years, with 44% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 45% (range: 12% to 91%), and mean postbronchodilator FEV1/FVC ratio was 46% (range: 17% to 81%), indicating that the patient population had moderate to very severely impaired airflow obstruction. The mean percent reversibility was 15% (range: -65% to 313%).
Patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg had a lower annual rate of moderate/severe COPD exacerbations compared with vilanterol in both trials (Table 7).
|
Treatment |
n |
Mean Annual Rate (exacerbations/year) |
Ratio vs. Vilanterol |
95% CI |
|
Trial 3 |
||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
403 |
0.90 |
0.79 |
0.64, 0.97 |
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
409 |
0.79 |
0.69 |
0.56, 0.85 |
|
Fluticasone furoate/vilanterol 50/25 mcg |
412 |
0.92 |
0.81 |
0.66, 0.99 |
|
Vilanterol 25 mcg |
409 |
1.14 |
–– |
–– |
|
Trial 4 |
||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
403 |
0.70 |
0.66 |
0.54, 0.81 |
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
402 |
0.90 |
0.85 |
0.70, 1.04 |
|
Fluticasone furoate/vilanterol 50/25 mcg |
408 |
0.92 |
0.87 |
0.72, 1.06 |
|
Vilanterol 25 mcg |
409 |
1.05 |
–– |
–– |
Comparator Trials
Three 12-week, randomized, double-blind, double-dummy trials were conducted with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily versus fluticasone propionate/salmeterol 250/50 mcg twice daily to evaluate the efficacy of serial lung function of fluticasone furoate/vilanterol ELLIPTA in patients with COPD. The primary endpoint of each trial was change from baseline in weighted mean FEV1 (0 to 24 hours) on Day 84. Of the 519 patients in Trial 5 (NCT01323634), 64% were male and 97% were White; mean age was 61 years; average smoking history was 40 pack years, with 55% identified as current smokers. At screening in the treatment group using fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 19% to 70%), the mean (SD) FEV1/FVC ratio was 0.51 (0.11), and the mean percent reversibility was 11% (range: -12% to 83%). At screening in the treatment group using fluticasone propionate/salmeterol 250/50 mcg, the mean postbronchodilator percent predicted FEV1 was 47% (range: 14% to 71%), the mean (SD) FEV1/FVC ratio was 0.49 (0.10), and the mean percent reversibility was 11% (range: -13% to 50%).
Of the 511 patients in Trial 6 (NCT01323621), 68% were male and 94% were White; mean age was 62 years; average smoking history was 35 pack years, with 52% identified as current smokers. At screening in the treatment group using fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 18% to 70%), the mean (SD) FEV1/FVC ratio was 0.51 (0.10), and the mean percent reversibility was 12% (range: -56% to 77%). At screening in the treatment group using fluticasone propionate/salmeterol 250/50 mcg, the mean postbronchodilator percent predicted FEV1 was 49% (range: 15% to 70%), the mean (SD) FEV1/FVC ratio was 0.50 (0.10), and the mean percent reversibility was 12% (range: -66% to 72%).
Of the 828 patients in Trial 7 (NCT01706328), 72% were male and 98% were White; mean age was 61 years; average smoking history was 38 pack years, with 60% identified as current smokers. At screening in the treatment group using fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 18% to 70%), the mean (SD) FEV1/FVC ratio was 0.52 (0.10), and the mean percent reversibility was 12% (range: -26% to 84%). At screening in the treatment group using fluticasone propionate/salmeterol 250/50 mcg, the mean postbronchodilator percent predicted FEV1 was 48% (range: 16% to 70%), the mean (SD) FEV1/FVC ratio was 0.51 (0.10), and the mean percent reversibility was 12% (range: -15% to 67%).
In Trial 5, the mean SE change from baseline in weighted mean FEV1 (0 to 24 hours) with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was 174 (15) mL compared with 94 (16) mL with fluticasone propionate/salmeterol 250/50 mcg (treatment difference 80 mL; 95% CI: 37, 124; P<0.001). In Trials 6 and 7, the mean (SE) change from baseline in weighted mean FEV1 (0 to 24 hours) with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was 142 (18) mL and 168 (12) mL, respectively, compared with 114 (18) mL and 142 (12) mL, respectively, for fluticasone propionate/salmeterol 250/50 mcg (Trial 6 treatment difference 29 mL; 95% CI: -22, 80; P = 0.267; Trial 7 treatment difference 25 mL; 95% CI: -8, 59; P = 0.137).
Mortality Trial
A randomized, double-blind, multicenter, multinational trial (NCT01313676) prospectively evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo on survival. The trial was event-driven, and patients were followed until a sufficient number of deaths occurred. In this trial, 16,568 patients aged 40 to 80 years received fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (n = 4,140), fluticasone furoate 100 mcg (n = 4,157), vilanterol 25 mcg (n = 4,140), or placebo (n = 4,131). Patients were treated for up to 4 years, with a median treatment duration of 1.5 years. Median duration of follow-up for the endpoint of survival was 1.8 years for all treatment groups. All patients had COPD with moderate airflow limitation (≥50% and ≤70% predicted FEV1) and either had a history of, or were at risk of, cardiovascular disease. The primary endpoint was all-cause mortality. Secondary efficacy endpoints included the rate of decline in FEV1, annual rate of moderate/severe COPD exacerbations, and health-related quality of life as measured by the St. George’s Respiratory Questionnaire for COPD patients (SGRQ-C).
Survival: Survival with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was not significantly improved compared with placebo (hazard ratio 0.88; 95% CI: 0.74, 1.04). Mortality per 100 patient-years was 3.1 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 3.5 for placebo, 3.2 for fluticasone furoate, and 3.4 for vilanterol.
Lung Function: A reduction of 8 mL/year was estimated on-treatment for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo in the rate of lung function decline as measured by FEV1 (95% CI: 1, 15).
Exacerbations: Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the on-treatment annual rate of moderate/severe exacerbations by 29% compared with placebo (95% CI: 22, 35). Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the annual rate of moderate/severe exacerbations by 19% compared with fluticasone furoate (95% CI: 12, 26) and by 21% compared with vilanterol (95% CI: 14, 28). The on-treatment annual rate of moderate/severe exacerbations was 0.25 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 0.35 for placebo, 0.31 for fluticasone furoate, and 0.31 for vilanterol.
Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the on-treatment annual rate of severe exacerbations (i.e., requiring hospitalization) by 27% compared with placebo (95% CI: 13, 39). Treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg reduced the on-treatment annual rate of exacerbations requiring hospitalization by 11% compared with fluticasone furoate (95% CI: -6, 25) and by 9% compared with vilanterol (95% CI: -8, 24).
Health-Related Quality of Life: The St. George’s Respiratory Questionnaire (SGRQ) is a disease-specific patient-reported instrument that measures symptoms, activities, and impact on daily life. The SGRQ-C, a shorter version derived from the original SGRQ, was used in this trial. Results were transformed to the SGRQ for reporting purposes. In a subset of 4,443 patients, the on-treatment SGRQ responder rates at 1 year (defined as a change in score of 4 or more as threshold) were 49% for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, 47% for placebo, 48% for fluticasone furoate, and 48% for vilanterol (odds ratio 1.18; 95% CI: 0.97, 1.44 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo).
14.2 Asthma
Adult Patients
The efficacy of fluticasone furoate/vilanterol ELLIPTA for the maintenance treatment of asthma was based on data from 4 randomized, double-blind, parallel-group clinical trials (Trial 8 [NCT01165138], Trial 9 [NCT01686633], Trial 10 [NCT01134042] and Trial 12 [NCT01086384]). While these 4 trials enrolled pediatric patients 12 to 17 years of age, these trials only support efficacy in adults [see Use in Specific Populations (8.4)]. In addition, patients in these trials were treated with fluticasone furoate/vilanterol ELLIPTA 200/25 mcg once daily by oral inhalation, which is not the approved recommended dosage for pediatric patients 12 years of age and older [see Dosage and Administration (2.2)]. Trials 8, 9, and 10 were designed to evaluate the safety and efficacy of fluticasone furoate/vilanterol ELLIPTA given once daily in patients who were not controlled on their current treatments of ICS or combination therapy consisting of an ICS plus a LABA. Trial 12 (24- to 76-week exacerbation trial) was designed to demonstrate that treatment with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg significantly decreased the risk of asthma exacerbations as measured by time to first asthma exacerbation when compared with fluticasone furoate 100 mcg. This trial enrolled patients who had 1 or more asthma exacerbations in the year prior to trial entry. The demographics of these 4 trials and the comparator trial (Trial 13, NCT01147848) are provided in Table 8.
| N/A = Data not collected. | |||||
| a Trials did not include current smokers; past smokers had fewer than 10 packs per year history. | |||||
|
Parameter |
Trial 8 n = 609 |
Trial 9 n = 1,039 |
Trial 10 n = 586 |
Trial 12 n = 2,019 |
Trial 13 n = 806 |
|
Mean age (years) (range) for all patients |
40 (12, 84) |
46 (12, 82) |
46 (12, 76) |
42 (12, 82) |
43 (12, 80) |
|
Mean age (years) (range) for adult patients |
44 (18, 84) |
48 (18, 82) |
47 (18, 76) |
46 (18, 82) |
46 (18, 80) |
|
Female (%) |
58 |
60 |
59 |
67 |
61 |
|
White (%) |
84 |
88 |
84 |
73 |
59 |
|
Duration of asthma (years) |
12 |
18 |
16 |
16 |
21 |
|
Never smokeda (%) |
N/A |
84 |
N/A |
86 |
81 |
|
Predose FEV1 (L) at baseline |
2.32 |
1.97 |
2.15 |
2.20 |
2.03 |
|
Mean percent predicted FEV1 at baseline (%) |
70 |
62 |
67 |
72 |
68 |
|
% Reversibility |
29 |
30 |
29 |
24 |
28 |
|
Absolute reversibility (mL) |
614 |
563 |
571 |
500 |
512 |
Trials 8, 9, and 10 were 12- or 24-week trials that evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA on lung function in patients with asthma. In Trial 8, patients were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate 100 mcg, or placebo. In Trial 9, patients were randomized to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg, fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, or fluticasone furoate 100 mcg. In Trial 10, patients were randomized to fluticasone furoate/vilanterol ELLIPTA 200/25 mcg, fluticasone furoate 200 mcg, or fluticasone propionate 500 mcg. All inhalations were administered once daily, with the exception of fluticasone propionate, which was administered twice daily. Patients receiving an ICS or an ICS plus a LABA (doses of ICS varied by trial and asthma severity) entered a 4-week run-in period during which LABA treatment was stopped. Patients reporting symptoms and/or rescue beta2‑agonist medication use during the run‑in period were continued in the trial.
In Trials 8 and 10, change from baseline in weighted mean FEV1 (0 to 24 hours) and change from baseline in trough FEV1 at approximately 24 hours after the last dose at study endpoint (12 and 24 weeks, respectively) were co-primary efficacy endpoints. In Trial 9, change from baseline in weighted mean FEV1 (0 to 24 hours) at Week 12 was the primary efficacy endpoint; change from baseline in trough FEV1 at approximately 24 hours after the last dose at Week 12 was a secondary endpoint. Table 9 provides the change from baseline in weighted mean FEV1 in mL (0 to 24 hours) and trough FEV1 in mL at study endpoint. Weighted mean FEV1 (0 to 24 hours) was derived from serial measurements taken within 30 minutes prior to dosing and post-dose assessments at 5, 15, and 30 minutes and 1, 2, 3, 4, 5, 12, 16, 20, 23, and 24 hours after the final dose. Other secondary endpoints included change from baseline in percentage of rescue-free 24-hour periods and percentage of symptom-free 24-hour periods over the treatment period.
| FEV1 = Forced Expiratory Volume in 1 second, ICS = Inhaled Corticosteroid, LABA = Long-acting Beta2-adrenergic Agonist. a Although these trials included pediatric patients 12 to 17 years of age, they only support the efficacy in adult patients. |
||||||||||
|
Study (Duration) Background Treatment |
n |
Weighted Mean FEV1 (0-24 h) (mL) |
||||||||
|
Difference from |
||||||||||
|
Treatment |
Placebo (95% CI) |
Fluticasone Furoate 100 mcg (95% CI) |
Fluticasone Furoate 200 mcg (95% CI) |
|||||||
|
Trial 8 (12 Weeks) Low- to mid-dose ICS or low-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
108 |
302 (178, 426) |
116 (-5, 236) |
–– |
||||||
|
Trial 9 (12 Weeks) Mid- to high-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
312 |
–– |
108 (45, 171) |
–– |
||||||
|
Trial 10 (24 Weeks) High-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
89 |
–– |
–– |
136 (1, 270) |
||||||
|
Study (Duration) Background Treatment |
n |
Trough FEV1 (mL) |
||||||||
|
Difference from |
||||||||||
|
Treatment |
Placebo (95% CI) |
Fluticasone Furoate 100 mcg (95% CI) |
Fluticasone Furoate 200 mcg (95% CI) |
|||||||
|
Trial 8 (12 Weeks) Low- to mid-dose ICS or low-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
200 |
172 (87, 258) |
36 (-48, 120) |
–– |
||||||
|
Trial 9 (12 Weeks) Mid- to high-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 100/25 mcg |
334 |
–– |
77 (16, 138) |
–– |
||||||
|
Trial 10 (24 Weeks) High-dose ICS or mid-dose ICS + LABA |
||||||||||
|
Fluticasone furoate/vilanterol ELLIPTA 200/25 mcg |
187 |
–– |
–– |
193 (108, 277) |
||||||
In Trial 8, weighted mean FEV1 (0 to 24 hours) was assessed in a subset of patients (n = 309). At Week 12, change from baseline in weighted mean FEV1 (0 to 24 hours) was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo (302 mL; 95% CI: 178, 426; P<0.001) (Table 9); change from baseline in weighted mean FEV1 (0 to 24 hours) for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was numerically greater than fluticasone furoate 100 mcg, but not statistically significant (116 mL; 95% CI: -5, 236). At Week 12, change from baseline in trough FEV1 was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with placebo (172 mL; 95% CI: 87, 258; P<0.001) (Table 9); change from baseline in trough FEV1 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was numerically greater than fluticasone furoate 100 mcg, but not statistically significant (36 mL; 95% CI: -48, 120).
In Trial 9, the change from baseline in weighted mean FEV1 (0 to 24 hours) was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg (108 mL; 95% CI: 45, 171; P<0.001) at Week 12 (Table 9). In a descriptive analysis, the change from baseline in weighted mean FEV1 (0 to 24 hours) for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg was numerically greater than fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (24 mL; 95% CI: -37, 86) at Week 12. The change from baseline in trough FEV1 was significantly greater for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg (77 mL, 95% CI: 16, 138; P = 0.014) at Week 12 (Table 9). In a descriptive analysis, the change from baseline in trough FEV1 for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg was numerically greater than fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (16 mL; 95% CI: -46, 77) at Week 12.
In Trial 10, the change from baseline in weighted mean FEV1 (0 to 24 hours) was significantly greater for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg compared with fluticasone furoate 200 mcg (136 mL; 95% CI: 1, 270; P = 0.048) at Week 24 (Table 9). The change from baseline in trough FEV1 was significantly greater for fluticasone furoate/vilanterol ELLIPTA 200/25 mcg compared with fluticasone furoate 200 mcg (193 mL, 95% CI: 108, 277; P<0.001) at Week 24.
Lung function improvements were demonstrated through weighted mean FEV1 (0 to 24 hours) over the 24-hour period following the final dose of fluticasone furoate/vilanterol ELLIPTA in Trials 9 and 10. Serial FEV1 measurements were taken within 30 minutes prior to dosing and postdose assessments at 5, 15, and 30 minutes and 1, 2, 3, 4, 5, 12, 16, 20, 23, and 24 hours in Trials 1, 2, and 3. A representative figure is shown from Trial 9 in Figure 4.
Figure 4. Least Squares (LS) Mean Change from Baseline in Individual Serial FEV1 (mL) Assessments over 24 Hours after 12 Weeks of Treatment (Trial 9)a
a Although these trials included pediatric patients 12 to 17 years of age, the data only support the efficacy in adult patients.
Patients receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg (Trial 9) or fluticasone furoate/vilanterol ELLIPTA 200/25 mcg (Trial 10) had significantly greater improvements from baseline in percentage of 24-hour periods without need of beta2-agonist rescue medication use and percentage of 24-hour periods without asthma symptoms compared with patients receiving fluticasone furoate 100 mcg or fluticasone furoate 200 mcg, respectively. In a descriptive analysis (Trial 9), patients receiving fluticasone furoate/vilanterol ELLIPTA 200/25 mcg had numerical improvements from baseline in percentage of 24-hour periods without need of beta2‑agonist rescue medication use and percentage of 24-hour periods without asthma symptoms compared with patients receiving fluticasone furoate/vilanterol ELLIPTA 100/25 mcg.
Trial 12 was a 24- to 76-week event-driven exacerbation trial that evaluated whether fluticasone furoate/vilanterol ELLIPTA 100/25 mcg significantly decreased the risk of asthma exacerbations as measured by time to first asthma exacerbation when compared with fluticasone furoate 100 mcg in patients with asthma. Patients receiving low- to high-dose ICS (fluticasone propionate 100 mcg to 500 mcg twice daily or equivalent) or low- to mid-dose ICS plus a LABA (fluticasone propionate/salmeterol 100/50 mcg to 250/50 mcg twice daily or equivalent) and a history of 1 or more asthma exacerbations that required treatment with oral/systemic corticosteroid or emergency department visit or in-patient hospitalization for the treatment of asthma in the year prior to trial entry, entered a 2-week run-in period during which LABA treatment was stopped. Patients reporting symptoms and/or rescue beta2-agonist medication use during the run-in period were continued in the trial.
The primary endpoint was time to first asthma exacerbation. Asthma exacerbation was defined as deterioration of asthma requiring the use of systemic corticosteroid for at least 3 days or an in‑patient hospitalization or emergency department visit due to asthma that required systemic corticosteroid. Rate of asthma exacerbation was a secondary endpoint. The hazard ratio from the Cox Model for the analysis of time to first asthma exacerbation for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg was 0.795 (95% CI: 0.642, 0.985). This represents a 20% reduction in the risk of experiencing an asthma exacerbation for patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg (P = 0.036). Mean yearly rates of asthma exacerbations of 0.14 and 0.19 in patients treated with fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg, respectively, were observed (25% reduction in rate; 95% CI: 5%, 40%).
Comparator Trial
Trial 13 was a 24-week trial that compared the efficacy of fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily with fluticasone propionate/salmeterol 250/50 mcg twice daily (N = 806). Patients receiving mid-dose ICS (fluticasone propionate 250 mcg twice daily or equivalent) entered a 4‑week run-in period during which all patients received fluticasone propionate 250 mcg twice daily. The primary endpoint was change from baseline in weighted mean FEV1 (0 to 24 hours) at Week 24.
The mean change (SE) from baseline in weighted mean FEV1 (0 to 24 hours) for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg was 341 (18.4) mL compared with 377 (18.5) mL for fluticasone propionate/salmeterol 250/50 mcg (treatment difference -37 mL; 95% CI: -88, 15; P = 0.162).
Pediatric Patients Aged 5 to 17 Years
The efficacy of fluticasone furoate/vilanterol ELLIPTA for the maintenance treatment of asthma in pediatric patients aged 5 to 17 years of age was based on Trial 14 (NCT03248128), a 24-week, randomized, double-blind, stratified, parallel-group clinical trial. This trial evaluated the efficacy of fluticasone furoate/vilanterol ELLIPTA compared with fluticasone furoate in 902 pediatric patients with asthma aged 5 to 17 years who were uncontrolled on their current ICS treatment. All inhalations were administered once daily in the morning. At trial entry patients had at least a 6-month history of asthma and had been receiving stable asthma therapy for at least 4 weeks prior to screening. Patients had to have a pre-bronchodilator FEV1 >50% to ≤100% of predicted normal and demonstrate a ≥12% reversibility of FEV1 within 15 to 40 minutes following 2 to 4 inhalations of albuterol inhalation aerosol (or 1 nebulized treatment with albuterol solution). Exclusion criteria included a history of life-threatening asthma or any asthma exacerbation requiring the use of oral corticosteroids, systemic or depot corticosteroids, emergency department visit, or hospitalization within 6 weeks, 3 months, or 6 months of screening, respectively.
Patients entered a 4-week open-label run-in period during which all patients received fluticasone propionate 100 mcg twice daily. Patients reporting symptoms and/or rescue beta2-agonist medication use during the last week of the run-in period were continued in the trial and were stratified by age. Pediatric patients aged 12 to 17 years (n = 229) were randomized 1:1 to fluticasone furoate/vilanterol ELLIPTA 100/25 mcg once daily (n = 117) or fluticasone furoate 100 mcg once daily (n = 112). Pediatric patients aged 5 to 11 years (n = 673) were randomized 1:1 to fluticasone furoate/vilanterol ELLIPTA 50/25 mcg once daily (n = 337) or fluticasone furoate 50 mcg once daily (n = 336). The primary endpoint was weighted mean FEV1 (0 to 4 hours) at Week 12. Of the 902 patients, the mean age was 10.0 years, 61% were male, and 73% were White, 8% African American, 6% American Indian or Alaska Native, 6% Asian, and 7% Other. Lung function improvements based on the primary endpoint of weighted mean FEV1 (0 to 4 hours) are presented in Table 10.
| FEV1 = Forced Expiratory Volume in 1 second, LS = Least Squares, SE = Standard Error. | |||||||||||||
| a The dose of fluticasone furoate was 100 mcg once daily for pediatric patients aged 12 to 17 years and 50 mcg once daily for pediatric patients aged 5 to 11 years. | |||||||||||||
| b The dose of fluticasone furoate/vilanterol ELLIPTA was 100/25 mcg once daily for pediatric patients aged 12 to 17 years and 50/25 mcg once daily for pediatric patients aged 5 to 11 years. | |||||||||||||
|
Primary Endpoint |
Fluticasone Furoatea (N = 448) |
Fluticasone Furoate/Vilanterol ELLIPTAb (N = 454) |
|||||||||||
|
Weighted Mean FEV1 (0-4 h) (mL) |
n = 397 |
n = 394 |
|||||||||||
|
LS mean |
1999 |
2081 |
|||||||||||
|
LS mean change (SE) |
323 (16.4) |
406 (16.5) |
|||||||||||
|
Difference vs fluticasone furoate |
83 |
||||||||||||
|
(95% CI) |
(37, 129) |
||||||||||||
Difference in LS mean change from baseline at Week 12 for fluticasone furoate/vilanterol ELLIPTA 100/25 mcg compared with fluticasone furoate 100 mcg was 106 mL (95% CI: -8, 220) in pediatric patients 12 to 17 years of age, and difference in LS mean change from baseline at Week 12 for fluticasone furoate/vilanterol ELLIPTA 50/25 mcg compared with fluticasone furoate 50 mcg was 73 mL (95% CI: 28, 118) in pediatric patients 5 to 11 years of age.
16. How is Fluticasone and Vilanterol Inhalation Powder supplied
Fluticasone Furoate/Vilanterol ELLIPTA is supplied as a disposable light grey and pale blue plastic inhaler containing 2 foil strips, each with 30 blisters.
One strip contains fluticasone furoate (100 or 200 mcg per blister), and the other strip contains vilanterol (25 mcg per blister).
A blister from each strip is used to create 1 dose. The inhaler is packaged within a moisture‑protective foil tray with a desiccant and a peelable lid in the following packs:
NDC 66993-135-97 100/25 mcg 30 inhalations (60 blisters)
NDC 66993-136-97 200/25 mcg 30 inhalations (60 blisters)
Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store in a dry place away from direct heat or sunlight. Keep out of reach of children.
Fluticasone Furoate/Vilanterol ELLIPTA should be stored inside the unopened moisture‑protective foil tray and only removed from the tray immediately before initial use. Discard Fluticasone Furoate/Vilanterol ELLIPTA 6 weeks after opening the foil tray or when the counter reads “0” (after all blisters have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Asthma-Related Events
Inform patients with asthma that LABA when used alone increases the risk of asthma-related hospitalization or asthma-related death. Available data show that when ICS and LABA are used together, such as with Fluticasone Furoate/Vilanterol ELLIPTA, there is not a significant increase in the risk of these events. [See Warnings and Precautions (5.1).]
Not for Acute Symptoms
Inform patients that Fluticasone Furoate/Vilanterol ELLIPTA is not meant to relieve acute symptoms of COPD or asthma and extra doses should not be used for that purpose. Advise patients to treat acute symptoms with an inhaled, short-acting beta2-agonist such as albuterol. Provide patients with such medication and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
- •
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- •
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- •
- Significant decrease in lung function as outlined by the physician
Tell patients they should not stop therapy with Fluticasone Furoate/Vilanterol ELLIPTA without physician/provider guidance since symptoms may recur after discontinuation. [See Warnings and Precautions (5.2).]
Do Not Use Additional Long-Acting Beta2-Agonists
Instruct patients not to use other LABA for COPD and asthma. [See Warnings and Precautions (5.3).]
Oropharyngeal Candidiasis
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat it with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with Fluticasone Furoate/Vilanterol ELLIPTA, but at times therapy with Fluticasone Furoate/Vilanterol ELLIPTA may need to be temporarily interrupted under close medical supervision. Advise patients to rinse the mouth with water without swallowing after inhalation to help reduce the risk of thrush. [See Warnings and Precautions (5.4).]
Pneumonia
Patients with COPD have a higher risk of pneumonia; instruct them to contact their healthcare providers if they develop symptoms of pneumonia. [See Warnings and Precautions (5.5).]
Immunosuppression and Risk of Infections
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. [See Warnings and Precautions (5.6).]
Hypercorticism and Adrenal Suppression
Advise patients that Fluticasone Furoate/Vilanterol ELLIPTA may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, inform patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to Fluticasone Furoate/Vilanterol ELLIPTA. [See Warnings and Precautions (5.8).]
Paradoxical Bronchospasm
As with other inhaled medicines, Fluticasone Furoate/Vilanterol ELLIPTA can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue Fluticasone Furoate/Vilanterol ELLIPTA and contact their healthcare provider right away. [See Warnings and Precautions (5.10).]
Hypersensitivity Reactions, including Anaphylaxis
Advise patients that hypersensitivity reactions (e.g., anaphylaxis, angioedema, rash, urticaria) may occur after administration of Fluticasone Furoate/Vilanterol ELLIPTA. Instruct patients to discontinue Fluticasone Furoate/Vilanterol ELLIPTA if such reactions occur. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder medications containing lactose; therefore, patients with severe milk protein allergy should not use Fluticasone Furoate/Vilanterol ELLIPTA. [See Warnings and Precautions (5.11).]
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk. [See Warnings and Precautions (5.13).]
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including fluticasone furoate, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of children taking corticosteroids by any route. [See Warnings and Precautions (5.14).]
Glaucoma and Cataracts
Advise patients that long-term use of ICS may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations. [See Warnings and Precautions (5.15).]
Risks Associated with Beta-Agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. Instruct patients to consult a healthcare practitioner immediately should any of these signs and symptoms develop. [See Warnings and Precautions (5.12).]
ELLIPTA is a registered trademark of the GSK group of companies.
Manufactured for:
Prasco Laboratories
Mason, OH 45040 USA
Manufactured by:
GlaxoSmithKline
Durham, NC 27701
BRE-PS:3PI
|
PATIENT INFORMATION Fluticasone Furoate/Vilanterol ELLIPTA inhalation powder (floo-tik’-a-sone fyoor’-oh-ate vye-lan’-ter-ol elip’-ta) for oral inhalation use |
||
|
What is Fluticasone Furoate/Vilanterol ELLIPTA?
|
||
|
Do not use Fluticasone Furoate/Vilanterol ELLIPTA:
|
||
|
Before using Fluticasone Furoate/Vilanterol ELLIPTA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Fluticasone Furoate/Vilanterol ELLIPTA and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
||
|
How should I use Fluticasone Furoate/Vilanterol ELLIPTA? Read the step-by-step instructions for using Fluticasone Furoate/Vilanterol ELLIPTA at the end of this Patient Information.
|
||
|
What are the possible side effects of Fluticasone Furoate/Vilanterol ELLIPTA? Fluticasone Furoate/Vilanterol ELLIPTA can cause serious side effects, including:
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
Common side effects of Fluticasone Furoate/Vilanterol ELLIPTA include: COPD: |
||
|
|
|
|
Asthma: |
||
|
|
|
|
These are not all the possible side effects of Fluticasone Furoate/Vilanterol ELLIPTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store Fluticasone Furoate/Vilanterol ELLIPTA?
Keep Fluticasone Furoate/Vilanterol ELLIPTA and all medicines out of the reach of children. |
||
|
General information about the safe and effective use of Fluticasone Furoate/Vilanterol ELLIPTA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fluticasone Furoate/Vilanterol ELLIPTA for a condition for which it was not prescribed. Do not give Fluticasone Furoate/Vilanterol ELLIPTA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Fluticasone Furoate/Vilanterol ELLIPTA that is written for health professionals. |
||
|
What are the ingredients in Fluticasone Furoate/Vilanterol ELLIPTA? Active ingredients: fluticasone furoate, vilanterol trifenatate
Inactive ingredients: lactose monohydrate (contains milk proteins), magnesium stearate For more information about Fluticasone Furoate/Vilanterol ELLIPTA, call 1-866-525-0688 or visit our website at www.Prasco.com. Manufactured for: |
||
- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: August 2023
- This Instructions for Use has been approved by the U.S. Food and Drug Administration Revised: August 2023
Principal Display Panel
NDC 66993-135-97
Fluticasone Furoate/Vilanterol
ELLIPTA Inhalation Powder
100 mcg/25 mcg
PRASCO
FOR ORAL INHALATION ONLY
Fluticasone Furoate/Vilanterol ELLIPTA Inhalation Powder contains 2 foil strips of 30 blisters each. Each blister on one strip contains 100 mcg of fluticasone furoate and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
Rx Only
1 ELLIPTA Inhaler containing 30 doses
(60 blisters total)
- 62000000089833 Rev. 08/23
Principal Display Panel
NDC 66993-136-97
Fluticasone Furoate/Vilanterol
ELLIPTA Inhalation Powder
200 mcg/25 mcg
PRASCO
FOR ORAL INHALATION ONLY
Fluticasone Furoate/Vilanterol ELLIPTA Inhalation Powder contains 2 foil strips of 30 blisters each. Each blister on one strip contains 200 mcg of fluticasone furoate and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
Rx Only
1 ELLIPTA Inhaler containing 30 doses
(60 blisters total)
- 62000000089828 Rev. 08/23
| FLUTICASONE FUROATE AND VILANTEROL
fluticasone furoate and vilanterol powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| FLUTICASONE FUROATE AND VILANTEROL
fluticasone furoate and vilanterol powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Prasco Laboratories (065969375) |
| Registrant - GlaxoSmithKline LLC (167380711) |
Frequently asked questions
More about fluticasone / vilanterol
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (228)
- Side effects
- Dosage information
- During pregnancy
- Drug class: bronchodilator combinations
- En español