FluMist: Package Insert / Prescribing Info
Package insert / product label
Generic name: influenza vaccine live
Dosage form: nasal spray
Drug class: Viral vaccines
Medically reviewed by Drugs.com. Last updated on Aug 27, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
FluMist (Influenza Vaccine Live, Intranasal)
Nasal Spray, for intranasal use
2025-2026 Formula
Initial U.S. Approval: 2003
Indications and Usage for FluMist
FluMist Dosage and Administration
Dosage Forms and Strengths
FluMist is a nasal spray. A single dose is 0.2 mL. (3)
Contraindications
- •
- Do not administer FluMist to persons who have had a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine including egg protein, or after a previous dose of any influenza vaccine. (4.1, 11)
- •
- Do not administer FluMist to children and adolescents through 17 years of age who are receiving aspirin therapy or aspirin-containing therapy. (4.2, 7.1)
Warnings and Precautions
- •
- In clinical trials, risks of hospitalization and wheezing were increased in children younger than 2 years of age who received FluMist. (5.1)
- •
- Children younger than 5 years of age with recurrent wheezing and persons of any age with asthma may be at increased risk of wheezing following the administration of FluMist. (5.2)
- •
- If Guillain-Barré syndrome has occurred within 6 weeks of any prior influenza vaccination, the decision to give FluMist should be based on careful consideration of the potential benefits and risks. (5.3)
Adverse Reactions/Side Effects
The most common solicited adverse reactions (≥10% in vaccine recipients and at least 5% greater than in placebo recipients) reported after FluMist were runny nose or nasal congestion (ages 2 years through 49 years), fever over 100°F (children ages 2 years through 6 years), and sore throat (adults ages 18 years through 49 years). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact MedImmune at 1-877-633-4411 or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
Drug Interactions
Antiviral drugs that are active against influenza A and/or B may reduce the effectiveness of FluMist if administered within 48 hours before, or within 2 weeks after, receipt of the vaccine. (7.2)
Use In Specific Populations
In clinical trials, in children 6 through 23 months of age, FluMist was associated with an increased risk of hospitalization and wheezing. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2025
Full Prescribing Information
1. Indications and Usage for FluMist
FluMist is a vaccine indicated for active immunization for the prevention of influenza disease caused by influenza virus subtypes A and type B contained in the vaccine [see Description (11)].
FluMist is approved for use in persons 2 through 49 years of age.
2. FluMist Dosage and Administration
For intranasal use.
2.1 Dosing Information
Administer FluMist according to the following schedule:
|
Age |
Dose |
Schedule |
|
2 years through 8 years |
If 2 doses, administer at least 1 month apart |
|
|
9 years through 49 years |
1 dose, 0.2 mL† |
- |
|
“-” indicates information is not applicable. |
||
2.2 Administration Instructions
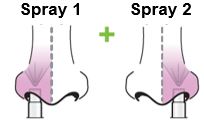
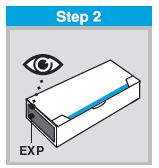
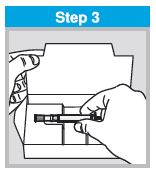
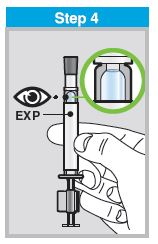
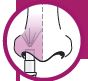
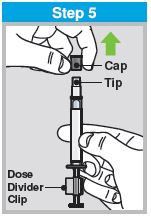
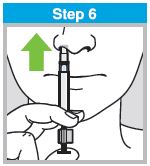
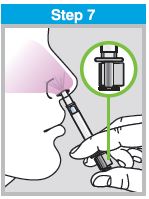
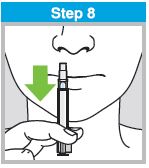
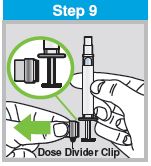
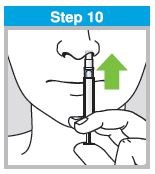
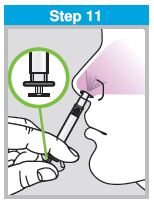
Each sprayer contains a single dose (0.2 mL) of FluMist; administer approximately one half of the contents of the single-dose intranasal sprayer into each nostril.
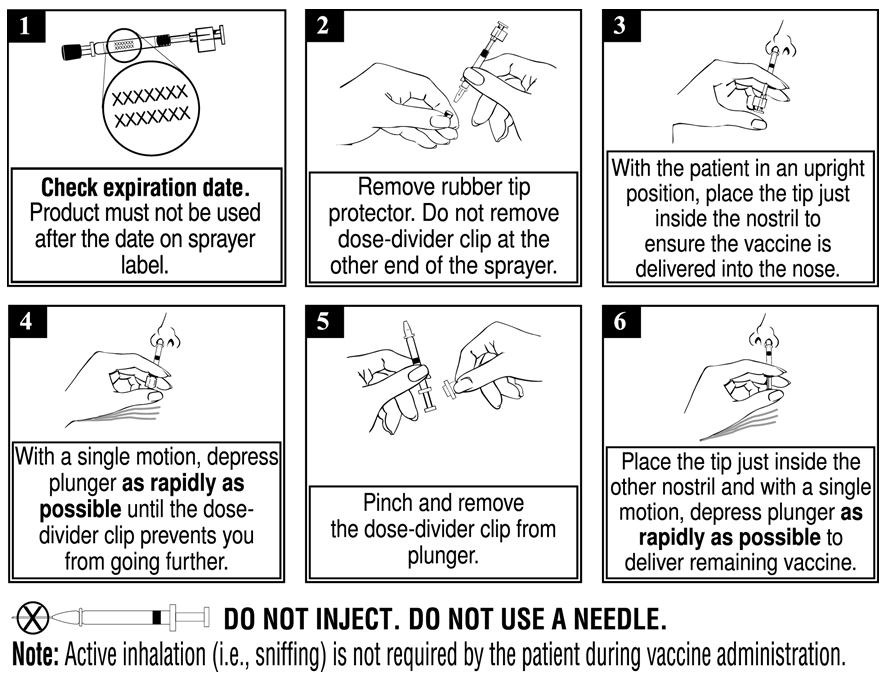
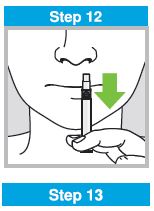
When administered by a healthcare provider in a healthcare setting, refer to Figure 1 for step-by-step administration instructions. Following administration, dispose of the sprayer according to the standard procedures for medical waste (e.g., sharps container or biohazard container).
Instruct vaccine recipients or caregivers who administer FluMist to follow the directions provided in the “Instructions for Use” for administration and disposal of FluMist. Individuals 2 through 17 years of age should not self‑administer FluMist.
Figure 1
4. Contraindications
4.1 Severe Allergic Reactions
Do not administer FluMist to persons who have had a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)] including egg protein, or after a previous dose of any influenza vaccine.
4.2 Concomitant Aspirin Therapy and Reye’s Syndrome in Children and Adolescents
Do not administer FluMist to children and adolescents through 17 years of age who are receiving aspirin therapy or aspirin-containing therapy because of the association of Reye’s syndrome with aspirin and wild-type influenza infection [see Drug Interactions (7.1)].
5. Warnings and Precautions
5.1 Risks of Hospitalization and Wheezing in Children Younger than 24 Months of Age
In clinical trials, risks of hospitalization and wheezing were increased in children younger than 2 years of age who received FluMist [see Adverse Reactions (6.1)].
5.2 Asthma, Recurrent Wheezing, and Active Wheezing
Children younger than 5 years of age with recurrent wheezing and persons of any age with asthma may be at increased risk of wheezing following administration of FluMist. FluMist has not been studied in persons with severe asthma or active wheezing.
5.3 Guillain-Barré Syndrome
If Guillain-Barré syndrome (GBS) has occurred within 6 weeks of any prior influenza vaccination, the decision to give FluMist should be based on careful consideration of the potential benefits and potential risks.
The 1976 swine influenza vaccine (inactivated) was associated with an elevated risk of GBS. Evidence for causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, based on data for inactivated influenza vaccines, it is probably slightly more than 1 additional case per 1 million persons vaccinated1.
5.4 Altered Immunocompetence
The effectiveness of FluMist has not been studied in immunocompromised persons. Data on safety and shedding of vaccine virus after administration of FluMist in immunocompromised persons are limited to 173 persons with HIV infection and 10 mild to moderately immunocompromised children and adolescents with cancer [see Clinical Pharmacology (12.2)].
5.5 Medical Conditions Predisposing to Influenza Complications
The safety of FluMist in individuals with underlying medical conditions that may predispose them to complications following wild-type influenza infection has not been established.
5.6 Management of Acute Allergic Reactions
When administered by a healthcare provider in a healthcare setting, appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of FluMist [see Error! Hyperlink reference not valid.].
When FluMist is self-administered or administered by a caregiver, immediate medical attention should be sought if the vaccine recipient experiences symptoms of an allergic reaction following administration of FluMist.
6. Adverse Reactions/Side Effects
The most common solicited adverse reactions (≥10% in vaccine recipients and at least 5% greater than in placebo recipients) reported after FluMist were runny nose or nasal congestion (ages 2 years through 49 years), fever over 100°F (children ages 2 years through 6 years), and sore throat (adults ages 18 years through 49 years).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
A total of 9537 children and adolescents 1 through 17 years of age and 3041 adults 18 through 64 years of age received FluMist in randomized, placebo-controlled Studies D153-P501, AV006, D153-P526, AV019, and AV009 [3 used Allantoic Fluid containing Sucrose-Phosphate-Glutamate (AF-SPG) placebo, and 2 used saline placebo] described below. In addition, 4179 children 6 through 59 months of age received FluMist in Study MI-CP111, a randomized, active-controlled trial. Among pediatric FluMist recipients 6 months through 17 years of age, 50% were female; in the study of adults, 55% were female. In MI-CP111, AV006, D153-P526, AV019, and AV009, subjects were White (71%), Hispanic (11%), Asian (7%), Black (6%), and Other (5%), while in D153-P501, 99% of subjects were Asian.
FluMist in Children and Adolescents
The safety of FluMist was evaluated in an AF-SPG placebo-controlled Study (AV019) conducted in a Health Maintenance Organization (HMO) in children 1 through 17 years of age (FluMist = 6473, placebo = 3216). An increase in asthma events, captured by review of diagnostic codes, was observed in children younger than 5 years of age who received FluMist compared to those who received placebo (Relative Risk 3.53, 90% CI: 1.1, 15.7).
In Study MI-CP111, children 6 through 59 months of age were randomized to receive FluMist or inactivated Influenza Virus Vaccine manufactured by Sanofi Pasteur Inc. Wheezing requiring bronchodilator therapy or accompanied by respiratory distress or hypoxia was prospectively monitored from randomization through 42 days post last vaccination. Hospitalization due to all causes was prospectively monitored from randomization through 180 days post last vaccination. Increases in wheezing and hospitalization (for any cause) were observed in children 6 months through 23 months of age who received FluMist compared to those who received inactivated Influenza Virus Vaccine, as shown in Table 1.
|
|||
|
Adverse Reaction |
Age Group |
FluMist
|
Active Control†
|
|
Hospitalizations‡
|
6-23 months |
4.2% |
3.2% |
|
24-59 months |
2.1% |
2.5% |
|
|
Wheezing§
|
6-23 months |
5.9% |
3.8% |
|
24-59 months |
2.1% |
2.5% |
|
Most hospitalizations observed were due to gastrointestinal and respiratory tract infections and occurred more than 6 weeks post vaccination. In post-hoc analysis, rates of hospitalization in children 6 through 11 months of age were 6.1% (42/684) in FluMist recipients and 2.6% (18/683) in inactivated Influenza Virus Vaccine recipients.
Table 2 shows pooled solicited adverse reactions occurring in at least 1% of FluMist recipients and at a higher rate (≥1% rate difference after rounding) compared to placebo post Dose 1 for Studies D153-P501 and AV006, and solicited adverse reactions post Dose 1 for Study MI-CP111. Solicited adverse reactions were those about which parents/guardians were specifically queried after receipt of FluMist, placebo, or control vaccine. In these studies, solicited reactions were documented for 10 days post vaccination. Solicited reactions following the second dose of FluMist were similar to those following the first dose and were generally observed at a lower frequency.
|
||||
|
Studies D153-P501* & AV006 |
Study MI-CP111† |
|||
|
FluMist |
Placebo‡ |
FluMist |
Active Control§ |
|
|
N = 876-1759¶ |
N = 424-1034¶ |
N = 2170¶ |
N = 2165¶ |
|
|
Event |
% |
% |
% |
% |
|
Runny Nose/ |
|
|
|
|
|
Decreased Appetite |
21 |
17 |
13 |
12 |
|
Irritability |
21 |
19 |
12 |
11 |
|
Decreased Activity |
14 |
11 |
7 |
6 |
|
Sore Throat |
11 |
9 |
5 |
6 |
|
Headache |
9 |
7 |
3 |
3 |
|
Muscle Aches |
6 |
3 |
2 |
2 |
|
Chills |
4 |
3 |
2 |
2 |
|
Fever | ||||
|
> 100°F Oral |
16 |
11 |
13 |
11 |
In clinical studies D153-P501 and AV006, unsolicited adverse reactions in children occurring in at least 1% of FluMist recipients and at a higher rate (≥1% rate difference after rounding) compared to placebo were abdominal pain (2% FluMist vs. 0% placebo) and otitis media (3% FluMist vs. 1% placebo). An additional adverse reaction identified in the active-controlled trial MI-CP111 occurring in at least 1% of FluMist recipients and at a higher rate (≥1% rate difference after rounding) compared to active control was sneezing (2% FluMist vs.1% active control).
In a separate saline placebo-controlled trial (D153-P526) in a subset of older children and adolescents 9 through 17 years of age who received one dose of FluMist, the solicited adverse reactions as well as unsolicited adverse reactions reported were generally consistent with observations from the trials in Table 2. Abdominal pain was reported in 12% of FluMist recipients compared to 4% of placebo recipients and decreased activity was reported in 6% of FluMist recipients compared to 0% of placebo recipients.
In Study AV018, in which FluMist was concomitantly administered with Measles, Mumps, and Rubella Virus Vaccine Live (MMR, manufactured by Merck & Co., Inc.) and Varicella Virus Vaccine Live (manufactured by Merck & Co., Inc.) to children 12 through 15 months of age, adverse reactions were similar to those seen in other clinical trials of FluMist.
FluMist in Adults
In adults 18 through 49 years of age in Study AV009, solicited adverse reactions occurring in at least 1% of FluMist recipients and at a higher rate (≥1% rate difference after rounding) compared to AF-SPG placebo include runny nose (44% FluMist vs. 27% placebo), headache (40% FluMist vs. 38% placebo), sore throat (28% FluMist vs. 17% placebo), tiredness/weakness (26% FluMist vs. 22% placebo), muscle aches (17% FluMist vs. 15% placebo), cough (14% FluMist vs. 11% placebo), and chills (9% FluMist vs. 6% placebo).
In Study AV009, unsolicited adverse reactions occurring in at least 1% of FluMist recipients and at a higher rate (≥1% rate difference after rounding) compared to placebo were nasal congestion (9% FluMist vs. 2% placebo) and sinusitis (4% FluMist vs. 2% placebo).
6.2 Postmarketing Experience
The following events have been spontaneously reported during post approval use of FluMist or FluMist Quadrivalent. Data for FluMist Quadrivalent are relevant to FluMist because both vaccines are manufactured using the same process and have overlapping compositions. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac disorders: Pericarditis
Congenital, familial, and genetic disorders: Exacerbation of symptoms of mitochondrial encephalomyopathy (Leigh syndrome)
Gastrointestinal disorders: Nausea, vomiting, diarrhea
Immune system disorders: Hypersensitivity reactions (including anaphylactic reaction, facial edema, and urticaria)
Nervous system disorders: Guillain-Barré syndrome, Bell’s Palsy, meningitis, eosinophilic meningitis, vaccine-associated encephalitis, syncope
Respiratory, thoracic, and mediastinal disorders: Epistaxis
Skin and subcutaneous tissue disorders: Rash
Related/similar drugs
7. Drug Interactions
7.1 Aspirin Therapy
Do not administer FluMist to children and adolescents through 17 years of age who are receiving aspirin therapy or aspirin-containing therapy because of the association of Reye’s syndrome with aspirin and wild-type influenza [see Contraindications (4.2)]. Avoid aspirin-containing therapy in these age groups during the first 4 weeks after vaccination with FluMist unless clearly needed.
7.2 Antiviral Agents Against Influenza A and/or B
Antiviral drugs that are active against influenza A and/or B viruses may reduce the effectiveness of FluMist if administered within 48 hours before, or within 2 weeks after vaccination. The concurrent use of FluMist with antiviral agents that are active against influenza A and/or B viruses has not been evaluated. If antiviral agents and FluMist are administered concomitantly, revaccination should be considered when appropriate.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
FluMist is not absorbed systemically following intranasal administration and maternal use is not expected to result in fetal exposure to the drug.
8.2 Lactation
Risk Summary
FluMist is not absorbed systemically by the mother following intranasal administration and breastfeeding is not expected to result in exposure of the child to FluMist.
8.4 Pediatric Use
FluMist is not approved for use in children younger than 24 months of age because use of FluMist in children 6 through 23 months has been associated with increased risks of hospitalization and wheezing in clinical trials [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
The effectiveness of FluMist in children 6 years through 17 years of age is supported by demonstration of efficacy in younger children 6 through 71 months of age and effectiveness in adults 18 through 49 years of age [see Clinical Studies (14)].
8.5 Geriatric Use
FluMist is not approved for use in persons 65 years of age and older because in a clinical study (AV009), effectiveness of FluMist to prevent febrile illness was not demonstrated in adults 50 through 64 years of age [see Clinical Studies (14.2)]. In this study, solicited events among individuals 50 through 64 years of age were similar in type and frequency to those reported in younger adults. In a clinical study of FluMist in persons 65 years of age and older, subjects with underlying high-risk medical conditions (N = 200) were studied for safety. Compared to controls, FluMist recipients had a higher rate of sore throat.
11. FluMist Description
FluMist (Influenza Vaccine Live, Intranasal) is a nasal spray. FluMist contains three vaccine virus strains: an A/H1N1 strain, an A/H3N2 strain and a B strain from the B/Victoria lineage.
The influenza virus strains in FluMist are (a) cold-adapted (ca) (i.e., they replicate efficiently at 25°C, a temperature that is restrictive for replication of many wild-type influenza viruses); (b) temperature-sensitive (ts) (i.e., they are restricted in replication at 37°C (Type B strain) or 39°C (Type A strains), temperatures at which many wild-type influenza viruses grow efficiently); and (c) attenuated (att) (i.e., they do not produce classic influenza-like illness in the ferret model of human influenza infection).
No evidence of reversion has been observed in the recovered vaccine strains that have been tested (135 of possible 250 recovered isolates) using FluMist [see Clinical Pharmacology (12.2)]. For each of the three reassortant strains in FluMist, the six internal gene segments responsible for ca, ts, and att phenotypes are derived from a master donor virus (MDV), and the two segments that encode the two surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), are derived from the corresponding antigenically relevant wild-type influenza viruses. Thus, the three viruses contained in FluMist maintain the replication characteristics and phenotypic properties of the MDV and express the HA and NA of wild-type viruses. For the Type A MDV, at least five genetic loci in three different internal gene segments contribute to the ts and att phenotypes. For the Type B MDV, at least three genetic loci in two different internal gene segments contribute to both the ts and att properties; five genetic loci in three gene segments control the ca property.
Each of the reassortant strains in FluMist express the HA and NA of wild- type viruses that are related to strains expected to circulate during the 2025-2026 influenza season. The viruses (A/H1N1, A/H3N2 and the B strain) have been recommended by the United States Public Health Service (USPHS) for inclusion in the annual trivalent influenza vaccine formulation.
Specific pathogen-free (SPF) eggs are inoculated with each of the reassortant strains and incubated to allow vaccine virus replication. The allantoic fluid of these eggs is harvested, pooled, and then clarified by filtration. The virus is concentrated by ultracentrifugation and diluted with stabilizing buffer to obtain the final sucrose and potassium phosphate concentrations. The viral harvests are then sterile filtered to produce the monovalent bulks. Each lot is tested for ca, ts, and att phenotypes and is also tested extensively by in vitro and in vivo methods to detect adventitious agents. Monovalent bulks from the three strains are subsequently blended and diluted as required to attain the desired potency with stabilizing buffers to produce the trivalent bulk vaccine. The bulk vaccine is then filled directly into individual sprayers for nasal administration.
Each pre-filled refrigerated FluMist sprayer contains a single 0.2 mL dose. Each 0.2 mL dose contains 106.5-7.5 FFU (fluorescent focus units) of live attenuated influenza virus reassortants of each of the three strains: A/Norway/31694/2022 (H1N1) (an A/Victoria/4897/2022 (H1N1)pdm09 - like virus), A/Perth/722/2024 (H3N2) (an A/Croatia/10136RV/2023 (H3N2) - like virus), and B/Austria/1359417/2021 (B/Victoria lineage) (a B/Austria/1359417/2021 (B/Victoria lineage) - like virus). Each 0.2 mL dose also contains 0.188 mg/dose monosodium glutamate, 2.00 mg/dose hydrolyzed porcine gelatin, 2.42 mg/dose arginine, 13.68 mg/dose sucrose, 2.26 mg/dose dibasic potassium phosphate, and 0.96 mg/dose monobasic potassium phosphate. Each dose contains residual amounts of ovalbumin (<0.024 mcg/dose), and may also contain residual amounts of gentamicin sulfate (<0.015 mcg/mL), and ethylenediaminetetraacetic acid (EDTA) (< 2.3 mcg/dose). FluMist contains no preservatives.
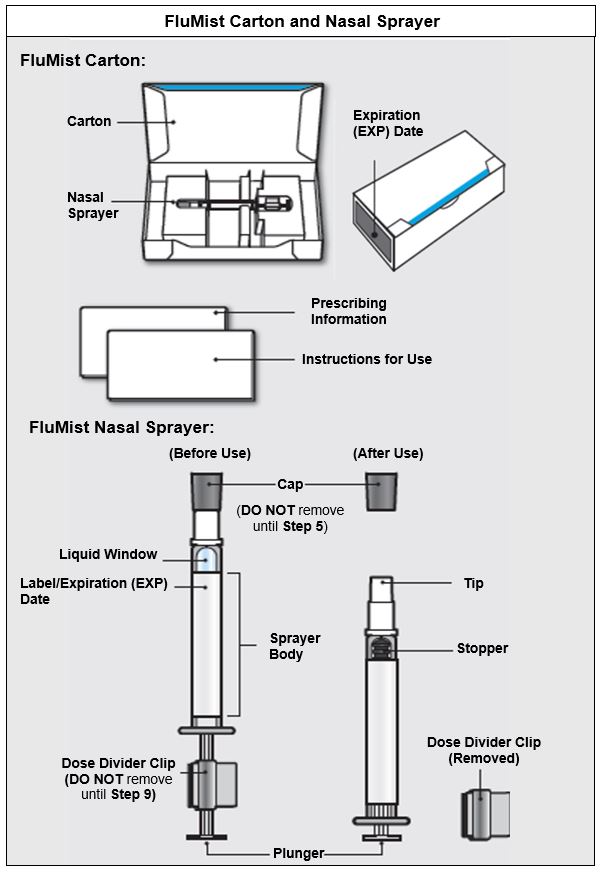
The tip attached to the sprayer is equipped with a nozzle that produces a fine mist that is primarily deposited in the nose and nasopharynx. FluMist is a colorless to pale yellow suspension and is clear to slightly cloudy.
12. FluMist - Clinical Pharmacology
12.1 Mechanism of Action
Immune mechanisms conferring protection against influenza following receipt of FluMist vaccine are not fully understood; serum antibodies, mucosal antibodies, and influenza-specific T cells may play a role.
FluMist contains live attenuated influenza viruses that must infect and replicate in cells lining the nasopharynx of the recipient to induce immunity. Vaccine viruses capable of infection and replication can be cultured from nasal secretions obtained from vaccine recipients (shedding) [see Clinical Pharmacology (12.2)].
12.2 Pharmacodynamics
Shedding Studies
Shedding of vaccine viruses within 28 days of vaccination with FluMist was evaluated in (1) multi-center Study MI‑CP129 which enrolled healthy individuals 6 through 59 months of age (N = 200); and (2) multi-center Study FM026 which enrolled healthy individuals 5 through 49 years of age (N = 344). In each study, nasal secretions were obtained daily for the first 7 days and every other day through either Day 25 and on Day 28 or through Day 28. In Study MI‑CP129, individuals with a positive shedding sample at Day 25 or Day 28 were to have additional shedding samples collected every 7 days until culture negative on 2 consecutive samples. Results of these studies are presented in Table 3.
|
|||||
|
Age |
Number of Subjects |
% Shedding‡ |
Peak Titer
|
%
|
Day of Last Positive Culture |
|
6-23 months¶
|
99 |
89 |
< 5 log10
|
7.0 | |
The highest proportion of subjects in each group shed one or more vaccine strains on Days 2-3 post vaccination. After Day 11 among individuals 2 through 49 years of age (n = 443), virus titers did not exceed 1.5 log10 TCID50/mL.
Studies in Immunocompromised Individuals
Safety and shedding of vaccine virus following FluMist administration were evaluated in 28 HIV‑infected adults [median CD4 cell count of 541 cells/mm3] and 27 HIV-negative adults 18 through 58 years of age. No serious adverse events were reported during the one-month follow-up period. Vaccine strain (type B) virus was detected in 1 of 28 HIV-infected subjects on Day 5 only, and in none of the HIV-negative FluMist recipients.
Safety and shedding of vaccine virus following FluMist administration were also evaluated in children in a randomized (1:1), cross-over, double-blind, AF-SPG placebo-controlled trial in 24 HIV-infected children [median CD4 cell count of 1013 cells/mm3] and 25 HIV-negative children 1 through 7 years of age, and in a randomized (1:1), open-label, inactivated influenza vaccine-controlled trial in 243 HIV-infected children and adolescents 5 through 17 years of age receiving stable anti-retroviral therapy. Frequency and duration of vaccine virus shedding in HIV-infected individuals were comparable to that seen in healthy individuals. No adverse effects on HIV viral load or CD4 counts were identified following FluMist administration. In the 5 through 17 year old age group, one inactivated influenza vaccine recipient and one FluMist recipient experienced pneumonia within 28 days of vaccination (days 17 and 13, respectively). The effectiveness of FluMist in preventing influenza illness in HIV-infected individuals has not been evaluated.
Twenty mild to moderately immunocompromised children and adolescents 5 through 17 years of age (receiving chemotherapy and/or radiation therapy or who had received chemotherapy in the 12 weeks prior to enrollment) were randomized 1:1 to receive FluMist or AF-SPG placebo. Frequency and duration of vaccine virus shedding in these immunocompromised children and adolescents were comparable to that seen in healthy children and adolescents. The effectiveness of FluMist in preventing influenza illness in immunocompromised individuals has not been evaluated.
Transmission Study
A prospective, randomized, double-blind, placebo-controlled trial was performed in a daycare setting in children younger than 3 years of age to assess the transmission of vaccine viruses from a vaccinated individual to a non-vaccinated individual. A total of 197 children 8 through 36 months of age were randomized to receive one dose of FluMist (N = 98) or AF-SPG placebo (N = 99). Virus shedding was evaluated for 21 days by culture of nasal swab specimens. Wild-type A (A/H3N2) influenza virus was documented to have circulated in the community and in the study population during the trial, whereas Type A (A/H1N1) and Type B strains did not.
At least one vaccine strain was isolated from 80% of FluMist recipients; strains were recovered from 1-21 days post vaccination (mean duration of 7.6 days ± 3.4 days). The ca and ts phenotypes were preserved in 135 tested of 250 strains isolated at the local laboratory. Ten influenza isolates (9 influenza A, 1 influenza B) were cultured from a total of seven placebo subjects. One placebo subject had mild symptomatic Type B virus infection confirmed as a transmitted vaccine virus by a FluMist recipient in the same playgroup. This Type B isolate retained the ca, ts, and att phenotypes of the vaccine strain and had the same genetic sequence when compared to a Type B virus cultured from a vaccine recipient within the same playgroup. Four of the influenza Type A isolates were confirmed as wild-type A/Panama (H3N2). The remaining isolates could not be further characterized.
Assuming a single transmission event (isolation of the Type B vaccine strain), the probability of a young child acquiring vaccine virus following close contact with a single FluMist vaccinee in this daycare setting was 0.58% (95% CI: 0, 1.7) based on the Reed-Frost model. With documented transmission of one Type B in one placebo subject and possible transmission of Type A viruses in four placebo subjects, the probability of acquiring a transmitted vaccine virus was estimated to be 2.4% (95% CI: 0.13, 4.6) using the Reed-Frost model.
14. Clinical Studies
14.1 Efficacy Studies of FluMist in Children
A multi-national, randomized, double-blind, active-controlled trial (MI-CP111) was performed to assess the efficacy of FluMist compared to an intramuscularly administered, inactivated Influenza Virus Vaccine manufactured by Sanofi Pasteur Inc. (active control) in children 6 months to less than 5 years of age during the 2004-2005 influenza season. A total number of 3916 children without severe asthma, without use of bronchodilator or steroids, and without wheezing within the prior 6 weeks were randomized to FluMist and 3936 were randomized to active control. Children who previously received any influenza vaccine received a single dose of study vaccine, while those who never previously received an influenza vaccination (or had an unknown history of influenza vaccination) received two doses. Participants were then followed through the influenza season to identify illness caused by influenza virus. As the primary endpoint, culture-confirmed modified CDC-ILI (CDC-defined influenza-like illness) was defined as a positive culture for a wild-type influenza virus associated within ±7 days of modified CDC-ILI. Modified CDC-ILI was defined as fever (temperature ≥100°F oral or equivalent) with cough, sore throat, or runny nose/nasal congestion on the same or consecutive days.
In the primary efficacy analysis, FluMist demonstrated a 44.5% (95% CI: 22.4, 60.6) reduction in influenza rate compared to active control as measured by culture-confirmed modified CDC-ILI caused by wild-type strains antigenically similar to those contained in the vaccine. See Table 4 for a description of the results by strain and antigenic similarity.
|
||||||||
|
|
FluMist |
Active Control§ |
%
|
95% CI |
||||
|
N |
# of
|
Rate
|
N |
# of
|
Rate
|
|||
|
Matched Strains | ||||||||
|
All strains |
3916 |
53 |
1.4% |
3936 |
93 |
2.4% |
44.5% |
22.4, 60.6 |
|
A/H1N1 |
3916 |
3 |
0.1% |
3936 |
27 |
0.7% |
89.2% |
67.7, 97.4 |
|
A/H3N2 |
3916 |
0 |
0.0% |
3936 |
0 |
0.0% |
-- |
-- |
|
B |
3916 |
50 |
1.3% |
3936 |
67 |
1.7% |
27.3% |
-4.8, 49.9 |
|
Mismatched Strains | ||||||||
|
All strains |
3916 |
102 |
2.6% |
3936 |
245 |
6.2% |
58.2% |
47.4, 67.0 |
|
A/H1N1 |
3916 |
0 |
0.0% |
3936 |
0 |
0.0% |
-- |
-- |
|
A/H3N2 |
3916 |
37 |
0.9% |
3936 |
178 |
4.5% |
79.2% |
70.6, 85.7 |
|
B |
3916 |
66 |
1.7% |
3936 |
71 |
1.8% |
6.3% |
-31.6, 33.3 |
|
Regardless of Match |
| |||||||
|
All strains |
3916 |
153 |
3.9% |
3936 |
338 |
8.6% |
54.9% |
45.4, 62.9 |
|
A/H1N1 |
3916 |
3 |
0.1% |
3936 |
27 |
0.7% |
89.2% |
67.7, 97.4 |
|
A/H3N2 |
3916 |
37 |
0.9% |
3936 |
178 |
4.5% |
79.2% |
70.6, 85.7 |
|
B |
3916 |
115 |
2.9% |
3936 |
136 |
3.5% |
16.1% |
-7.7, 34.7 |
|
ATP Population. |
||||||||
A randomized, double-blind, saline placebo-controlled trial (D153-P501) was performed to evaluate the efficacy of FluMist in children 12 through 35 months of age without high-risk medical conditions against culture-confirmed influenza illness. This study was performed in Asia over two successive seasons (2000-2001 and 2001-2002). The primary endpoint of the trial was the prevention of culture-confirmed influenza illness due to antigenically matched wild-type influenza. Respiratory illness that prompted an influenza culture was defined as at least one of the following: fever (≥100.4°F rectal or ≥99.5°F axillary), wheezing, shortness of breath, pulmonary congestion, pneumonia, or otitis media; or two of the following: runny nose/nasal congestion, sore throat, cough, muscle aches, chills, headache, irritability, decreased activity, or vomiting. A total of 3174 children were randomized 3:2 (vaccine:placebo) to receive 2 doses of study vaccine or placebo at least 28 days apart in Year 1. See Table 5 for a description of the results.
During the second year of Study D153-P501, for children who received two doses in Year 1 and one dose in Year 2, FluMist demonstrated 84.3% (95% CI: 70.1, 92.4) efficacy against culture-confirmed influenza illness due to antigenically matched wild-type influenza.
Study AV006 was a second multi-center, randomized, double-blind, AF-SPG placebo-controlled trial performed in U.S. children without high-risk medical conditions to evaluate the efficacy of FluMist against culture-confirmed influenza over two successive seasons (1996-1997 and 1997-1998). The primary endpoint of the trial was the prevention of culture-confirmed influenza illness due to antigenically matched wild-type influenza in children who received two doses of vaccine in the first year and a single revaccination dose in the second year. Respiratory illness that prompted an influenza culture was defined as at least one of the following: fever (≥101°F rectal or oral; or ≥100.4°F axillary), wheezing, shortness of breath, pulmonary congestion, pneumonia, or otitis media; or two of the following: runny nose/nasal congestion, sore throat, cough, muscle aches, chills, headache, irritability, decreased activity, or vomiting. During the first year of the study, 1602 children 15 through 71 months of age were randomized 2:1 (vaccine:placebo). See Table 5 for a description of the results.
|
||||||
|
|
D153-P501§ |
AV006¶ |
||||
|
|
FluMist
|
Placebo
|
% Efficacy
|
FluMist
|
Placebo
|
% Efficacy
|
|
|
NÞ = 1653 |
NÞ = 1111 |
|
NÞ = 849 |
NÞ = 410 |
|
|
Any strain |
56 (3.4%) |
139 (12.5%) |
72.9%ß
|
10 (1%) |
73 (18%) |
93.4% |
|
A/H1N1 |
23 (1.4%) |
81 (7.3%) |
80.9% |
0 |
0 |
-- |
|
A/H3N2 |
4 (0.2%) |
27 (2.4%) |
90.0% |
4 (0.5%) |
48 (12%) |
96.0% |
|
B |
29 (1.8%) |
35 (3.2%) |
44.3% |
6 (0.7%) |
31 (7%) |
90.5% |
During the second year of Study AV006, children remained in the same treatment group as in Year 1 and received a single dose of FluMist or placebo. During the second year, the primary circulating strain was the A/Sydney/05/97 H3N2 strain, which was antigenically dissimilar from the H3N2 strain represented in the vaccine, A/Wuhan/359/95; FluMist demonstrated 87.0% (95% CI: 77.0, 92.6) efficacy against culture-confirmed influenza illness.
14.2 Effectiveness Study of FluMist in Adults
AV009 was a U.S. multi-center, randomized, double-blind, AF-SPG placebo-controlled trial to evaluate effectiveness of FluMist in adults 18 through 64 years of age without high-risk medical conditions over the 1997-1998 influenza season. Participants were randomized 2:1 (vaccine:placebo). Cultures for influenza virus were not obtained from subjects in the trial, thus efficacy against culture-confirmed influenza was not assessed. The A/Wuhan/359/95 (H3N2) strain, which was contained in FluMist, was antigenically distinct from the predominant circulating strain of influenza virus during the trial period, A/Sydney/05/97 (H3N2). Type A/Wuhan (H3N2) and Type B strains also circulated in the U.S. during the study period. The primary endpoint of the trial was the reduction in the proportion of participants with one or more episodes of any febrile illness, and prospective secondary endpoints were severe febrile illness and febrile upper respiratory illness. Effectiveness for any of the three endpoints was not demonstrated in a subgroup of adults 50 through 64 years of age. Primary and secondary effectiveness endpoints from the age group 18 through 49 years are presented in Table 6. Effectiveness was not demonstrated for the primary endpoint in adults 18 through 49 years of age.
|
Endpoint |
FluMist
|
Placebo
|
Percent
|
(95% CI) |
|
Participants with one or more
|
|
|
|
|
Effectiveness was shown in a post-hoc analysis using an endpoint of CDC-ILI in the age group 18 through 49 years of age.
14.3 Concomitantly Administered Live Virus Vaccines
In Study AV018, concomitant administration of FluMist, MMR (manufactured by Merck & Co., Inc.) and Varicella Virus Vaccine Live (manufactured by Merck & Co., Inc.) was studied in 1245 subjects 12 through 15 months of age. Subjects were randomized in a 1:1:1 ratio to MMR, Varicella vaccine and AF-SPG placebo (group 1); MMR, Varicella vaccine and FluMist (group 2); or FluMist alone (group 3). Immune responses to MMR and Varicella vaccines were evaluated 6 weeks post-vaccination while the immune responses to FluMist were evaluated 4 weeks after the second dose. No evidence of interference with immune response to measles, mumps, rubella, varicella and FluMist vaccines was observed.
15. References
- 1.
- Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992 – 1993 and 1993 – 1994 influenza vaccines. N Engl J Med 1998;339(25):1797-802.
16. How is FluMist supplied
16.1 How Supplied
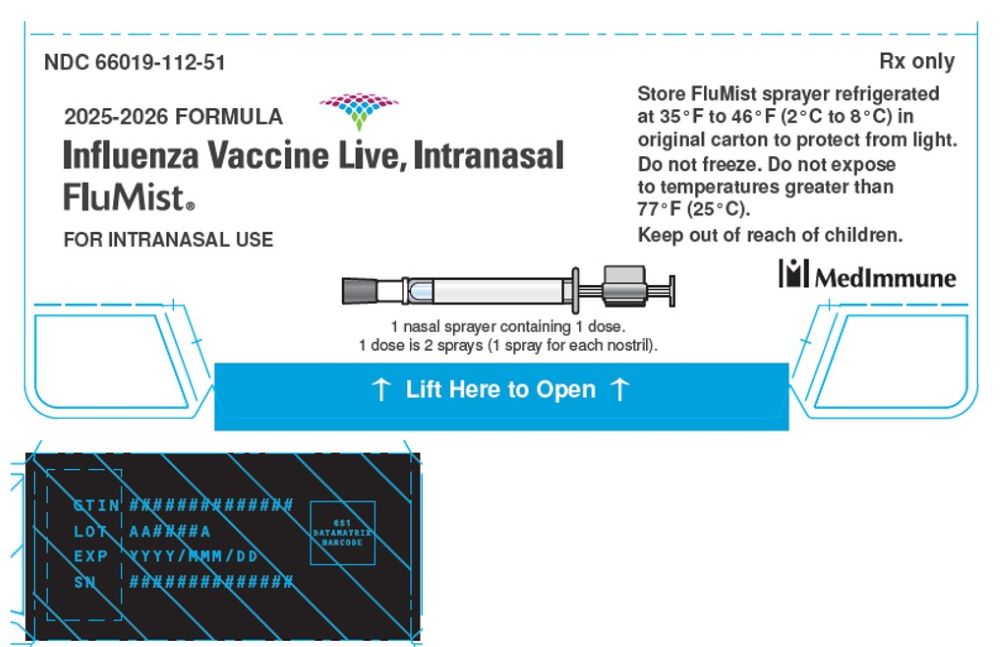
FluMist is supplied in a single-dose (0.2 mL) intranasal sprayer. The single-use intranasal sprayer is not made with natural rubber latex.
Single intranasal sprayer: NDC 66019-112-00
Carton containing 1 intranasal sprayer: NDC 66019-112-51
Carton containing 10 intranasal sprayers: NDC 66019-112-10
16.2 Storage and Handling
FLUMIST SHOULD BE STORED IN A REFRIGERATOR BETWEEN 2-8°C (35-46°F) UPON RECEIPT. THE PRODUCT MUST NOT BE USED AFTER THE EXPIRATION DATE ON THE SPRAYER LABEL.
DO NOT FREEZE.
Keep FluMist sprayer in outer carton in order to protect from light.
A single temperature excursion up to 25°C (77°F) for 12 hours has been shown to have no adverse impact on the vaccine. After a temperature excursion, the vaccine should be returned immediately to the recommended storage condition (2°C – 8°C [35 – 46°F]) and used as soon as feasible. Subsequent excursions are not permitted.
In a healthcare setting, when FluMist has been administered or has expired, the sprayer should be disposed of according to the standard procedures for medical waste (e.g., sharps container or biohazard container). Instruct vaccine recipients or caregivers who administer FluMist to follow the directions provided in the “Instructions for Use” for disposal of FluMist.
17. Patient Counseling Information
When FluMist is self-administered or administered by a caregiver, instruct the vaccine recipient or caregiver to read the “Instructions for Use.” Individuals 2 through 17 years of age should not self‑administer FluMist.
Advise the vaccine recipient or caregiver to read the FDA-approved patient labeling (Information for Patients and Their Caregivers).
Provide the Vaccine Information Statements (VIS) which are required by the National Childhood Vaccine Injury Act of 1986 to be given with each immunization.
Dosing in Children 2 through 8 years of age
Inform the caregiver of the need for two doses at least 1 month apart in children 2 through 8 years of age, depending on vaccination history.
Asthma and Recurrent Wheezing
Ask if the vaccine recipient has asthma. For children younger than 5 years of age, also ask if the vaccine recipient has recurrent wheezing since this may be an asthma equivalent in this age group. Inform the vaccine recipient or caregiver that there may be an increased risk of wheezing associated with FluMist in persons younger than 5 years of age with recurrent wheezing and persons of any age with asthma [see Warnings and Precautions (5.2)].
Vaccination with a Live Virus Vaccine
Inform the vaccine recipient or caregiver that FluMist is an attenuated live virus vaccine and has the potential for transmission to immunocompromised household contacts.
Acute Allergic Reactions
Instruct the vaccine recipient or caregiver to seek immediate medical attention if the vaccine recipient experiences any symptoms of an acute allergic reaction [see Warnings and Precautions (5.6)].
Adverse Event Reporting
Instruct the vaccine recipient or caregiver to report adverse reactions to their healthcare provider.
FluMist® is a registered trademark of MedImmune, LLC.
Manufactured by:
MedImmune, LLC
Gaithersburg, MD 20878
1-877-633-4411
U.S. Government License No. 1799
Issue Date: August 2025
Patient Package Insert
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
2025-2026 FORMULA
Influenza Vaccine Live, Intranasal
FluMist®
STORE REFRIGERATED
at 2º-8ºC (35º-46ºF)
RX only
For 2-49 years of age
PROTECT FROM LIGHT
FOR INTRANASAL USE
Contents: 10 pre-filled sprayers
One 0.2 mL dose each
(0.1 mL per nostril)
Manufactured by MedImmune, LLC.
One MedImmune Way
Gaithersburg, MD 20878 USA
U.S. Govt. License No. 1799
Product of the United Kingdom
MedImmune
Package/Label Display Panel
2025-2026 FORMULA
Influenza Vaccine Live, Intranasal
FluMist®
FOR INTRANASAL USE
1 nasal sprayer containing 1 dose.
1 dose is 2 sprays (1 spray for each nostril).
STORE FluMist sprayer refrigerated
at 35ºF to 46ºF (2ºC to 8ºC) in
original carton to protect from light.
Do not freeze. Do not expose
to temperatures greater than 77ºF (25ºC).
Keep out of reach of children.
Manufactured by MedImmune, LLC.
One MedImmune Way
Gaithersburg, MD 20878 USA
U.S. Govt. License No. 1799
Product of the United Kingdom
MedImmune
| FLUMIST
influenza vaccine live intranasal spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - MedImmune, LLC (190639906) |
Frequently asked questions
- How and where is a flu shot injection given?
- What flu vaccine can I use with an egg allergy?
- FluMist or Flu Shot: Which is more effective?
- Where can I get the flu vaccine right now?
- How well does the flu vaccine work?
- How can I get a flu vaccine without a needle?
- Which flu vaccines are available this year?
- When is the best month to get the flu vaccine?
- Can I get FluMist and the COVID vaccine at the same time?
More about FluMist (influenza virus vaccine, live)
- Check interactions
- Compare alternatives
- Reviews (14)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: viral vaccines