Fasenra: Package Insert / Prescribing Info
Package insert / product label
Generic name: benralizumab
Dosage form: injection, solution
Drug class: Interleukin inhibitors
J Code (medical billing code): J0517 (1 mg, injection)
Medically reviewed by Drugs.com. Last updated on Sep 30, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
FASENRA (benralizumab) injection, for subcutaneous use
Initial U.S. Approval: 2017
Recent Major Changes
Indications and Usage for Fasenra
FASENRA is an interleukin-5 receptor alpha-directed cytolytic monoclonal antibody (IgG1, kappa) indicated for:
- •
- add-on maintenance treatment of adult and pediatric patients aged 6 years and older with severe asthma, and with an eosinophilic phenotype. (1.1)
- •
- treatment of adult patients with eosinophilic granulomatosis with polyangiitis (EGPA). (1.2)
Limitations of Use:
Not for relief of acute bronchospasm or status asthmaticus. (1.1)
Fasenra Dosage and Administration
Administer by subcutaneous injection. (2.3)
Asthma
Adult and Adolescent Patients 12 Years of Age and Older:
- •
- Recommended dosage is 30 mg every 4 weeks for first 3 doses followed by once every 8 weeks thereafter. (2.1)
Pediatric Patients 6 Years to 11 Years of Age:
- •
- Weighing Less Than 35 kg: the recommended dosage is 10 mg every 4 weeks for first 3 doses followed by once every 8 weeks thereafter. (2.1)
- •
- Weighing 35 kg or More: the recommended dosage is 30 mg every 4 weeks for first 3 doses followed by once every 8 weeks thereafter. (2.1)
EGPA
Recommended dosage is 30 mg every 4 weeks. (2.2)
See full prescribing information for administration instructions of FASENRA prefilled syringe and FASENRA PEN. (2.4, 2.5)
Dosage Forms and Strengths
Contraindications
Known hypersensitivity to benralizumab or excipients. (4)
Warnings and Precautions
- •
- Hypersensitivity reactions: Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, rash) have occurred after administration of FASENRA. Discontinue in the event of a hypersensitivity reaction. (5.1)
- •
- Reduction in Corticosteroid Dosage: Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with FASENRA. Decrease corticosteroids gradually, if appropriate. (5.3)
- •
- Parasitic (Helminth) Infection: Treat patients with pre-existing helminth infections before therapy with FASENRA. If patients become infected while receiving FASENRA and do not respond to anti-helminth treatment, discontinue FASENRA until the parasitic infection resolves. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence greater than or equal to 5%) include headache and pharyngitis. (6.1, 6.2)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2024
Full Prescribing Information
1. Indications and Usage for Fasenra
1.1 Asthma
FASENRA is indicated for the add-on maintenance treatment of adult and pediatric patients aged 6 years and older with severe asthma, and with an eosinophilic phenotype [see Use in Specific Populations (8.4), Clinical Studies (14.1)].
Limitations of Use:
- •
- FASENRA is not indicated for the relief of acute bronchospasm or status asthmaticus.
2. Fasenra Dosage and Administration
2.1 Recommended Dosage for Asthma
Adult and Adolescent Patients 12 Years of Age and Older
The recommended dosage of FASENRA is 30 mg (one injection) administered subcutaneously every 4 weeks for the first 3 doses, and then every 8 weeks thereafter.
Pediatric Patients 6 to 11 Years of Age
The recommended dosage of FASENRA for pediatric patients 6 to 11 years of age is based on body weight as provided in Table 1.
|
Body weight |
Recommended Dosage |
|
Less than 35 kg |
10 mg (one injection) administered subcutaneously every 4 weeks for the first 3 doses, and then every 8 weeks thereafter. |
|
35 kg or more |
30 mg (one injection) administered subcutaneously every 4 weeks for the first 3 doses, and then every 8 weeks thereafter. |
2.2 Recommended Dosage for EGPA
The recommended dosage of FASENRA is 30 mg (one injection) administered once every 4 weeks by subcutaneous injection.
2.3 General Administration Instructions
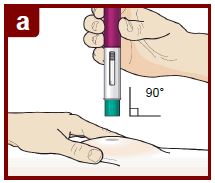
FASENRA is for subcutaneous use only.
FASENRA is intended for use under the guidance of a healthcare provider. In line with clinical practice, monitoring of patients after administration of biologic agents is recommended [see Warnings and Precautions (5.1)].
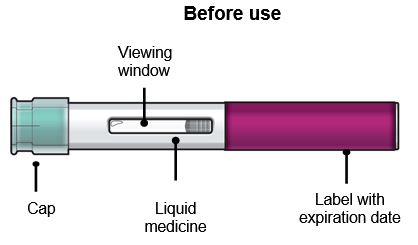
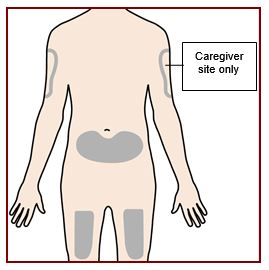
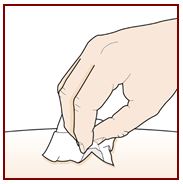
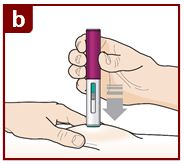
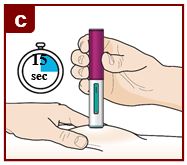
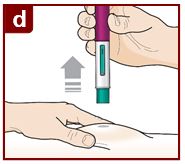
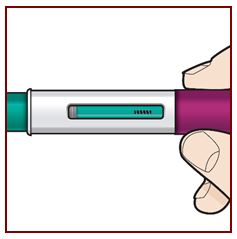
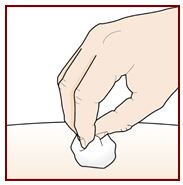
Administer FASENRA into the thigh or abdomen. The upper arm can also be used if a healthcare provider or caregiver administers the injection. Prior to administration, warm FASENRA by leaving carton at room temperature for about 30 minutes. Visually inspect FASENRA for particulate matter and discoloration prior to administration. FASENRA is clear to opalescent, colorless to slightly yellow, and may contain a few translucent or white to off-white particles. Do not use FASENRA if the liquid is cloudy, discolored, or if it contains large particles or foreign particulate matter.
Prefilled Syringe
The prefilled syringe is for administration by a healthcare provider.
Autoinjector (FASENRA PEN™)
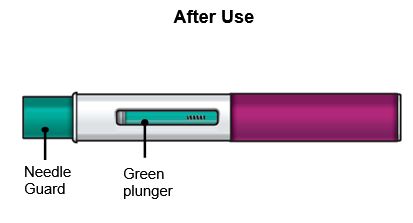
FASENRA PEN is intended for administration by patients/caregivers. Patients/caregivers may inject after proper training in subcutaneous injection technique, and after the healthcare provider determines it is appropriate.
In asthma patients aged 6 to 11 years weighing 35 kg or more, FASENRA PEN should only be administered by a caregiver or healthcare provider.
2.4 Instructions for Administration of FASENRA Prefilled Syringe (Healthcare Providers)
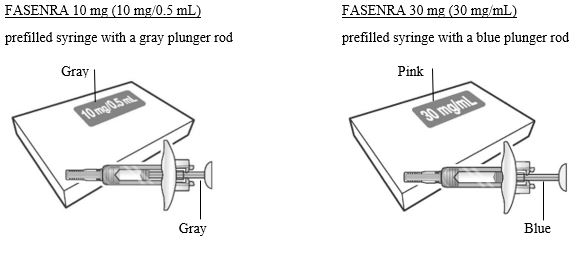
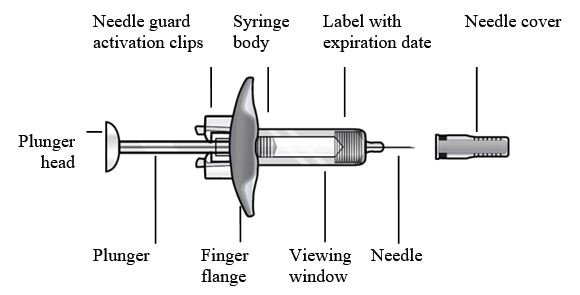
- Figure 1 FASENRA Prefilled Syringes
Prefilled Syringe Components
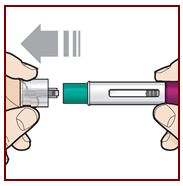
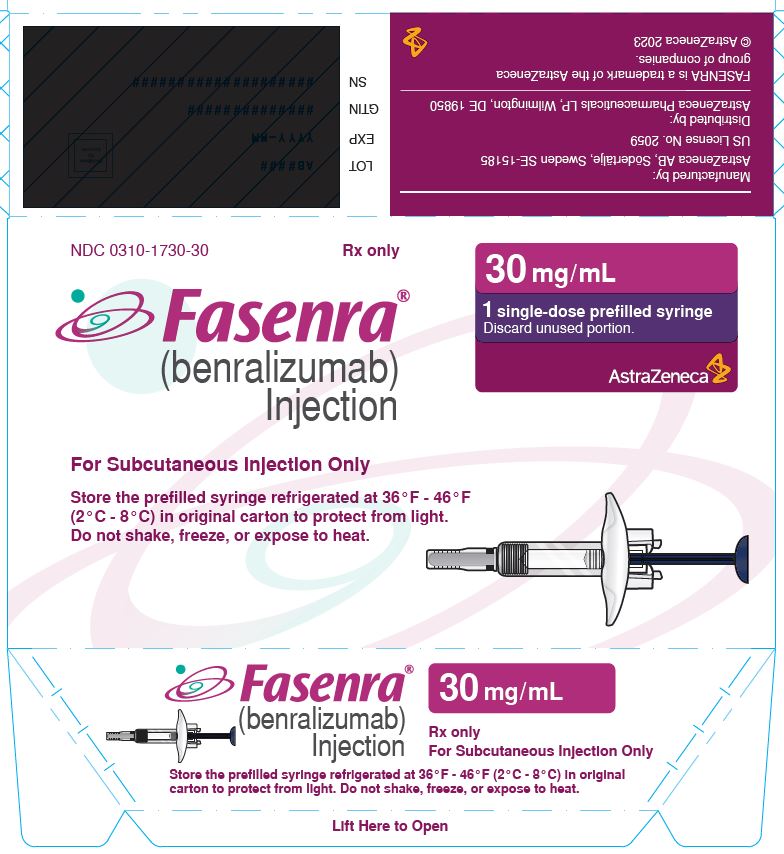
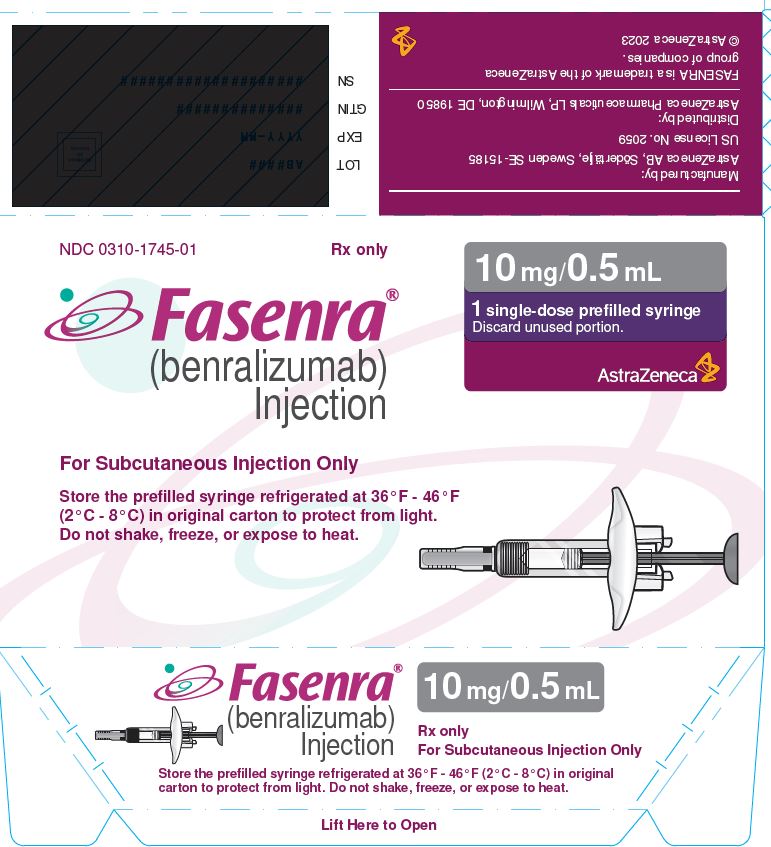
To prepare FASENRA prefilled syringe for subcutaneous administration, carefully read and adhere to these instructions for use. FASENRA is available in a 10 mg and a 30 mg prefilled syringe. Check the labels on the FASENRA carton and prefilled syringe to ensure the correct 10 mg or 30 mg product is being used (Figure 1). Refer to Figure 1 to identify the prefilled syringe components for use in the administration steps.
Do not touch the needle guard activation clips to prevent premature activation of the needle safety guard.
2.5 Instructions for Administration of FASENRA PEN
Refer to the FASENRA PEN ‘Instructions for Use’ for more detailed instructions on the preparation and administration of FASENRA PEN [see Instructions for Use]. A patient may self-inject or the patient’s caregiver may administer FASENRA PEN subcutaneously after the healthcare provider determines it is appropriate. In patients aged 6 to 11 years weighing 35 kg or more, FASENRA PEN should only be administered by a caregiver or healthcare provider.
3. Dosage Forms and Strengths
Injection: clear to opalescent, colorless to slightly yellow solution and may contain a few translucent or white to off‑white particles available as:
- •
- 10 mg/0.5 mL solution in a single-dose prefilled syringe.
- •
- 30 mg/mL solution in a single-dose prefilled syringe.
- •
- 30 mg/mL solution in a single-dose autoinjector FASENRA PEN.
4. Contraindications
FASENRA is contraindicated in patients who have known hypersensitivity to benralizumab or any of its excipients [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, rash) have occurred following administration of FASENRA. These reactions generally occur within hours of administration, but in some instances have a delayed onset (i.e., days). In the event of a hypersensitivity reaction, FASENRA should be discontinued [see Contraindications (4)].
5.2 Acute Asthma Symptoms or Deteriorating Disease
FASENRA should not be used to treat acute asthma symptoms or acute exacerbations. Do not use FASENRA to treat acute bronchospasm or status asthmaticus. Patients should seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with FASENRA.
5.3 Reduction of Corticosteroid Dosage
Do not discontinue systemic or inhaled corticosteroids (ICS) abruptly upon initiation of therapy with FASENRA. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
5.4 Parasitic (Helminth) Infection
Eosinophils may be involved in the immunological response to some helminth infections. Patients with known helminth infections were excluded from participation in clinical trials. It is unknown if FASENRA will influence a patient’s response against helminth infections.
Treat patients with pre-existing helminth infections before initiating therapy with FASENRA. If patients become infected while receiving treatment with FASENRA and do not respond to anti-helminth treatment, discontinue treatment with FASENRA until infection resolves.
6. Adverse Reactions/Side Effects
The following adverse reactions are described in greater detail in other sections:
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Adult and Adolescent Patients 12 Years of Age and Older with Asthma
Across three clinical trials (SIROCCO, CALIMA, and ZONDA) for asthma, 1,808 patients received at least 1 dose of FASENRA [see Clinical Studies (14.1)]. The data described below reflect exposure to FASENRA in 1,663 patients, including 1,556 exposed for at least 24 weeks and 1,387 exposed for at least 48 weeks. The safety exposure for FASENRA is derived from two Phase 3 placebo-controlled trials (SIROCCO and CALIMA) from 48 weeks duration [FASENRA every 4 weeks (n=841), FASENRA every 4 weeks for 3 doses, then every 8 weeks (n=822), and placebo (n=847)]. While a dosing regimen of FASENRA every 4 weeks was included in clinical trials, FASENRA administered every 4 weeks for 3 doses, then every 8 weeks thereafter is the recommended dose [see Dosage and Administration (2.1)]. The population studied was 12 to 75 years of age, of which 64% were female and 79% were White.
Adverse reactions that occurred at greater than or equal to 3% incidence are shown in Table 2.
|
||
|
Adverse Reactions |
FASENRA (N=822) % |
Placebo (N=847) % |
|
Headache |
8 |
6 |
|
Pyrexia |
3 |
2 |
|
Pharyngitis* |
5 |
3 |
|
Hypersensitivity reactions† |
3 |
3 |
28-Week Trial
Adverse reactions from ZONDA with 28 weeks of treatment with FASENRA (n=73) or placebo (n=75) in which the incidence was more common in FASENRA than placebo include headache (8.2% compared to 5.3%, respectively) and pyrexia (2.7% compared to 1.3%, respectively) [see Clinical Studies (14.1)]. The frequencies for the remaining adverse reactions with FASENRA were similar to placebo.
Injection Site Reactions in Patients with Asthma
In SIROCCO and CALIMA, the FASENRA 30 mg prefilled syringe was administered at the recommended dosage and injection site reactions (e.g., pain, erythema, pruritus, papule) occurred at a rate of 2.2% in patients treated with FASENRA compared with 1.9% in patients treated with placebo.
Pediatric Patients 6 to 11 Years of Age with Asthma
The safety data for FASENRA is based on a 48-week, open-label, parallel group, pharmacokinetic and pharmacodynamic trial (TATE) of 28 pediatric patients aged 6 to 11 years with severe asthma, and with an eosinophilic phenotype [see Use in Specific Populations (8.4) and Clinical Pharmacology (12.2,12.3)]. Patients received subcutaneous dose of 10 mg (for those weighing less than 35 kg) or 30 mg (for those weighing 35 kg or more) of FASENRA administered every 4 weeks for the first 3 doses, then every 8 weeks thereafter [see Dosage and Administration (2.1)]. No new safety signals were observed in these patients.
Adult Patients with EGPA
The safety of FASENRA is based on 70 adult patients who received at least 1 dose of FASENRA 30 mg administered subcutaneously every 4 weeks in an active-controlled study of 52 weeks duration (MANDARA) [see Clinical Studies (14.2)]. The incidence of adverse reactions were consistent to those reported in asthma, with the exception of headache, which occurred in 17% of FASENRA-treated patients with EGPA. No new adverse reactions were identified.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during post-approval use of FASENRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to FASENRA or a combination of these factors.
Immune System Disorders: Hypersensitivity reactions, including anaphylaxis.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
The data on pregnancy exposure from the clinical trials are insufficient to inform on drug-associated risk. Monoclonal antibodies such as benralizumab are transported across the placenta during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy. In a prenatal and postnatal development study conducted in cynomolgus monkeys, there was no evidence of fetal harm with IV administration of benralizumab throughout pregnancy at doses that produced exposures up to approximately 310 times the exposure at the maximum recommended human dose (MRHD) of 30 mg SC [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
In women with poorly or moderately controlled asthma, evidence demonstrates that there is an increased risk of preeclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. The level of asthma control should be closely monitored in pregnant women and treatment adjusted as necessary to maintain optimal control.
Data
Animal Data
In a prenatal and postnatal development study, pregnant cynomolgus monkeys received benralizumab from beginning on GD20 to GD22 (dependent on pregnancy determination), on GD35, once every 14 days thereafter throughout the gestation period and 1-month postpartum (maximum 14 doses) at doses that produced exposures up to approximately 310 times that achieved with the MRHD (on an AUC basis with maternal IV doses up to 30 mg/kg once every 2 weeks). Benralizumab did not elicit adverse effects on fetal or neonatal growth (including immune function) up to 6.5 months after birth. There was no evidence of treatment-related external, visceral, or skeletal malformations. Benralizumab was not teratogenic in cynomolgus monkeys. Benralizumab crossed the placenta in cynomolgus monkeys. Benralizumab concentrations were approximately equal in mothers and infants on postpartum day 7, but were lower in infants at later time points. Eosinophil counts were suppressed in infant monkeys with gradual recovery by 6 months postpartum; however, recovery of eosinophil counts was not observed for one infant monkey during this period.
8.2 Lactation
Risk Summary
There is no information regarding the presence of benralizumab in human or animal milk, and the effects of benralizumab on the breast fed infant and on milk production are not known. However, benralizumab is a humanized monoclonal antibody (IgG1/κ-class), and immunoglobulin G (IgG) is present in human milk in small amounts. If benralizumab is transferred into human milk, the effects of local exposure in the gastrointestinal tract and potential limited systemic exposure in the infant to benralizumab are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for benralizumab and any potential adverse effects on the breast-fed child from benralizumab or from the underlying maternal condition.
8.4 Pediatric Use
Asthma
The safety and effectiveness of FASENRA for add-on maintenance treatment of patients with severe asthma and with an eosinophilic phenotype have been established in pediatric patients 6 years and older. Use of FASENRA for this indication is supported by evidence from the following:
Adolescent Patients 12 to 17 Years of Age
Use of FASENRA in adolescents with severe asthma and with an eosinophilic phenotype is supported by evidence from SIROCCO (n=53) and CALIMA (n=55) that enrolled 108 adolescents aged 12 to 17 years (mean age 14 years, 42% female, White 82%, Asian 2%, Black or African American 4%) with asthma. Of these patients, 46 received placebo, 40 received 30 mg of FASENRA every 4 weeks for 3 doses, followed by every 8 weeks thereafter, and 22 received 30 mg of FASENRA every 4 weeks. Patients were required to weigh 40 kg or more and to have a history of 2 or more asthma exacerbations requiring oral or systemic corticosteroid treatment in the past 12 months and reduced lung function at baseline (pre-bronchodilator FEV1<90%) despite regular treatment with medium or high dose ICS and LABA with or without OCS or other controller therapy [see Clinical Studies (14)]. The pharmacokinetics of benralizumab in adolescents 12 to 17 years of age were consistent with adults based on population pharmacokinetic analysis and the reduction in blood eosinophil counts was similar to that observed in adults following the same FASENRA treatment. The adverse reaction profile in adolescents was generally similar to the overall population in the clinical trials [see Adverse Reactions (6.1)].
Pediatric Patients 6 to 11 Years of Age
Use of FASENRA in pediatric patients aged 6 to 11 years with severe asthma, and with an eosinophilic phenotype is supported by evidence from adequate and well-controlled trials in adults and adolescents with additional pharmacokinetic, pharmacodynamic, and safety data in pediatric patients aged 6 to 11 years. The effectiveness of FASENRA in pediatric patients 6 to 11 years of age is extrapolated from efficacy in three clinical trials (SIROCCO, CALIMA, and ZONDA) [see Clinical Studies (14)] with support from pharmacokinetic analysis and pharmacodynamic response in pediatric patients aged 6 to 11 years compared to adults and adolescents. TATE is a 48-week, open-label, pharmacokinetic and pharmacodynamic trial that was conducted in 28 patients aged 6 to 11 years (mean age 9 years; 6-8 years, n=11; 9-11 years n=17; 32% female, White 29%, Asian 32%, Black or African American 29%) with severe asthma, and with an eosinophilic phenotype.
Based upon the pharmacokinetic data from TATE, a subcutaneous dose of 10 mg (patients <35 kg) and subcutaneous dose of 30 mg (patients ≥35 kg) of benralizumab administered every 4 weeks for the first 3 doses, then every 8 weeks thereafter in patients aged 6 to 11 years was determined to have similar or higher exposure, respectively, to adults and adolescents administered a subcutaneous dose of 30 mg with the same dosing regimen [see Clinical Pharmacology (12.3)]. The pharmacodynamic response observed in TATE for pediatric patients aged 6 to 11 years was similar to that observed in adults and adolescents [see Clinical Pharmacology (12.2)]. No new safety signals were observed from TATE and safety for the higher drug exposure is supported by safety data from SIROCCO and CALIMA in adults and adolescents, and ZONDA in adults, who received 30 mg of FASENRA every 4 weeks for 1 year.
The safety and effectiveness in patients younger than 6 years of age have not been established.
EGPA
The safety and effectiveness of FASENRA in patients with EGPA younger than 18 years of age have not been established.
8.5 Geriatric Use
Asthma
Of the total number of patients in asthma clinical trials of benralizumab, 13% (n=320) were 65 and over, while 0.4% (n=9) were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
EGPA
Of the 70 patients with EGPA exposed to FASENRA, a total of 13 (19%) were 65 years or older. Clinical studies of FASENRA for EGPA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
10. Overdosage
There is no specific treatment for an overdosage with benralizumab. If overdose occurs, the patient should be treated supportively with appropriate monitoring as necessary.
11. Fasenra Description
Benralizumab is a humanized monoclonal antibody (IgG1/κ-class) selective for interleukin-5 receptor alpha subunit (IL-5Rα). Benralizumab is produced in Chinese hamster ovary cells by recombinant DNA technology. Benralizumab has a molecular weight of approximately 150 kDa.
FASENRA (benralizumab) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution for subcutaneous injection. Since benralizumab is a protein, a few translucent or white to off-white particles may be present in the solution.
Each 10 mg single-dose prefilled syringe delivers 0.5 mL containing 10 mg benralizumab, L-histidine (0.7 mg); L-histidine hydrochloride monohydrate (1.2 mg); polysorbate 20 (0.03 mg); α,α‑trehalose dihydrate (47 mg); and Water for Injection, USP, at pH of 5.5 – 6.5. The single-dose prefilled syringe contains a 1 mL glass syringe with a staked 29 gauge ½ inch stainless steel needle.
Each 30 mg single-dose prefilled syringe or single-dose autoinjector delivers 1 mL containing 30 mg benralizumab, L-histidine (1.4 mg); L-histidine hydrochloride monohydrate (2.3 mg); polysorbate 20 (0.06 mg); α,α‑trehalose dihydrate (95 mg); and Water for Injection, USP, at pH of 5.5 – 6.5. The single-dose prefilled syringe or single-dose autoinjector contains a 1 mL glass syringe with a staked 29 gauge ½ inch stainless steel needle.
12. Fasenra - Clinical Pharmacology
12.1 Mechanism of Action
Benralizumab is a humanized afucosylated, monoclonal antibody (IgG1, kappa) that directly binds to the alpha subunit of the human interleukin-5 receptor (IL-5Rα) with a dissociation constant of 11 pM. The IL-5 receptor is expressed on the surface of eosinophils and basophils. In an in vitro setting, the absence of fucose in the Fc domain of benralizumab facilitates binding (45.5 nM) to FcɣRIII receptors on immune effector cells, such as natural killer (NK) cells, leading to apoptosis of eosinophils and basophils through antibody-dependent cell-mediated cytotoxicity (ADCC).
Inflammation is an important component in the pathogenesis of asthma and EGPA. Multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) are involved in inflammation. Benralizumab, by binding to the IL-5Rα chain, reduces eosinophils through ADCC; however, the mechanism of benralizumab action in asthma and EGPA has not been definitively established.
12.2 Pharmacodynamics
In the 52-week Phase 2 dose-ranging trial, asthma patients received 1 of 3 doses of benralizumab [2 mg (n=81), 20 mg (n=81), or 100 mg (n=222)] or placebo (n=222). All doses were administered every 4 weeks for the first 3 doses, followed by every 8 weeks thereafter. Median blood eosinophil levels at baseline were 310, 280, 190 and 190 cells/μL in the 2, 20, and 100 mg benralizumab and placebo groups, respectively. Dose-dependent reductions in blood eosinophils were observed. At the time of the last dose (Week 40), median blood eosinophil counts were 100, 50, 40, 170 cells/μL in the 2, 20, and 100 mg benralizumab and placebo groups, respectively.
A reduction in blood eosinophil counts was observed 24 hours post dosing in an asthma Phase 2 trial.
In SIROCCO and CALIMA, following SC administration of benralizumab at the recommended dose blood eosinophils were reduced to a median absolute blood eosinophil count of 0 cells/μL [see Clinical Studies (14)]. This magnitude of reduction was observed at the first time point, 4 weeks of treatment, and was maintained throughout the treatment period.
Treatment with benralizumab was also associated with reductions in blood basophils, which was consistently observed across all asthma clinical studies. In the Phase 2 dose-ranging asthma trial, blood basophil counts were measured by flow cytometry. Median blood basophil counts were 45, 52, 46, and 40 cells/µL in the 2 mg, 20 mg and 100 mg benralizumab and placebo groups, respectively. At 52 weeks (12 weeks after the last dose), median blood basophil counts were 42, 18, 17, and 46 cells/µL in the 2 mg, 20 mg and 100 mg benralizumab and placebo groups, respectively.
In TATE, a 48-week trial with patients aged 6 to 11 years who had severe asthma, and with an eosinophilic phenotype [see Use in Specific Populations (8.4)], the magnitude of blood eosinophil reduction was similar to that observed in adults and adolescents. Median blood eosinophil levels at baseline were 400 and 340 cells/µL in patients weighing <35 kg and ≥35 kg, respectively. Across all post-dose time points, median eosinophil counts reduced to 10 to 20 cells/µL in patients weighing <35 kg, and to 20 to 30 cells/µL in patients weighing ≥35 kg. Blood eosinophil reduction was observed at the first time point, 4 weeks of treatment, and was maintained throughout the treatment period.
In patients with EGPA, reduction of blood eosinophils was consistent with the effect observed in asthma trials. Median absolute blood eosinophil levels in the benralizumab group at baseline were 240 cells/μL. Following SC administration of benralizumab at the recommended dosage in patients with EGPA, blood eosinophils were reduced to a median absolute blood eosinophil count of 20 to 30 cells/μL. Blood eosinophil reduction was seen at the first observed time point, 1 week of treatment, and was maintained throughout the 52‑week treatment period.
12.3 Pharmacokinetics
The pharmacokinetic properties of benralizumab below are based on the population pharmacokinetic analyses from the asthma trials. Findings in EGPA were generally consistent with those in asthma although a lower clearance is predicted for patients with EGPA relative to patients with asthma [see Elimination].
The pharmacokinetics of benralizumab was approximately dose-proportional in adult and adolescent patients with asthma following subcutaneous administration over a dose range of 20 to 200 mg.
Absorption
Following subcutaneous administration to patients with asthma, the absorption half-life was approximately 3.5 days. Based on population pharmacokinetic analysis, the estimated absolute bioavailability was approximately 59% and there was no clinically relevant difference in relative bioavailability in the administration to the abdomen, thigh, or arm.
Distribution
Based on population pharmacokinetic analysis, central and peripheral volume of distribution of benralizumab was 3.1 L and 2.5 L, respectively, for a 70 kg individual.
Elimination
From population pharmacokinetic analysis, benralizumab exhibited linear pharmacokinetics and no evidence of target receptor-mediated clearance pathway. The estimated typical systemic clearance (CL) for benralizumab was 0.29 L/d for an asthma patient weighing 70 kg. The estimated typical CL was 0.22 L/d for patients with EGPA. Following subcutaneous administration in patients with asthma, the elimination half-life was approximately 15.5 days.
Metabolism
Benralizumab is a humanized IgG1 monoclonal antibody that is degraded by proteolytic enzymes widely distributed in the body and not restricted to hepatic tissue.
Specific populations:
Age
Based on population pharmacokinetic analysis, age did not affect benralizumab clearance.
Gender, Race
A population pharmacokinetics analysis indicated that there was no significant effect of gender and race on benralizumab clearance.
Patients with Renal impairment
No formal clinical studies have been conducted to investigate the effect of renal impairment on benralizumab. Based on population pharmacokinetic analysis, benralizumab clearance was comparable in subjects with creatinine clearance values between 30 and 80 mL/min and patients with normal renal function. There are limited data available in subjects with creatinine clearance values less than 30 mL/min; however, benralizumab is not cleared renally.
Patients with Hepatic impairment
No formal clinical studies have been conducted to investigate the effect of hepatic impairment on benralizumab. IgG monoclonal antibodies are not primarily cleared via hepatic pathway; change in hepatic function is not expected to influence benralizumab clearance. Based on population pharmacokinetic analysis, baseline hepatic function biomarkers (ALT, AST, and bilirubin) had no clinically relevant effect on benralizumab clearance.
Pediatric Patients
Benralizumab pharmacokinetics following subcutaneous administration of 10 mg or 30 mg in patients aged 6 to 11 years with severe asthma and with an eosinophilic phenotype asthma was investigated in the initial 16-week treatment phase of TATE, a 48-week open-label trial. Among patients aged 6 to 11 years weighing <35 kg who received 10 mg, the median trough concentration at Week 16 was similar to that of adults and adolescents who received 30 mg. Among patients aged 6 to 11 years weighing ≥35 kg who received 30 mg, the median trough concentration at Week 16 was 62% higher relative to adults and adolescents receiving the same dose, due to lower body weight in pediatric patients.
Drug Interaction Studies
No formal drug-drug interaction studies have been conducted.
Cytochrome P450 enzymes, efflux pumps and protein-binding mechanisms are not involved in the clearance of benralizumab. There is no evidence of IL-5Rα expression on hepatocytes and eosinophil depletion does not produce chronic systemic alterations of proinflammatory cytokines.
An effect of benralizumab on the pharmacokinetics of co-administered medications is not expected. Based on the population analysis, commonly co-administered medications had no effect on benralizumab clearance in patients with asthma.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of benralizumab or of other benralizumab products.
In adult and adolescent patients with asthma, the incidence of ADA to benralizumab in patients treated with FASENRA at the recommended dosing regimen during the 48 to 56‑week treatment period was 13%. A total of 12% of patients treated with FASENRA developed neutralizing antibodies.
In pediatric patients 6 to 11 years with severe asthma and with an eosinophilic phenotype, the incidence of ADA to benralizumab during an open-label 48-week treatment period was comparable to adult and adolescent patients.
In patients with EGPA, the incidence of ADA to benralizumab during the active-controlled 52-week treatment period was 9% (6 out of 67 patients). Neutralizing antibody activity was detected in one of the ADA positive patients.
Anti-Drug Antibody Effects on Pharmacokinetics and Pharmacodynamics
Anti-benralizumab antibodies were associated with increased clearance of benralizumab and increased blood eosinophil levels in asthma patients with high ADA titers compared to antibody negative patients. Reduced benralizumab trough concentrations were observed in one EGPA patient with high ADA titers.
In the asthma trials with adult and adolescent patients and in the EGPA trial, no evidence of an association of ADA with efficacy or safety was observed.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of benralizumab. Published literature using animal models suggests that IL-5 and eosinophils are part of an early inflammatory reaction at the site of tumorigenesis and can promote tumor rejection. However, other reports indicate that eosinophil infiltration into tumors can promote tumor growth. Therefore, the malignancy risk in humans from an antibody that binds to IL-5Rα such as benralizumab is unknown.
Male and female fertility were unaffected based upon no adverse histopathological findings in the reproductive organs from cynomolgus monkeys treated with benralizumab for 9 months at IV doses up to 25 mg/kg or at SC doses of up to 30 mg/kg once every 2 weeks (approximately 400 and 270 times the MRHD on an AUC basis).
14. Clinical Studies
14.1 Clinical Studies in Patients with Asthma
The efficacy of FASENRA for the add-on maintenance treatment of severe asthma, and with an eosinophilic phenotype was evaluated in two randomized, double-blind, parallel-group, placebo-controlled, exacerbation trials, SIROCCO (NCT01928771) and CALIMA (NCT01914757), for 48 and 56 weeks in duration, respectively. Furthermore, the effects of FASENRA in the reduction of oral corticosteroid use and effect on lung function were evaluated in clinical trials, ZONDA (NCT02075255) and a 12-week lung function trial (NCT02322775), respectively.
SIROCCO and CALIMA were randomized, double-blind, parallel-group, placebo-controlled, exacerbation trials in patients 12 years of age and older and 48 and 56 weeks in duration, respectively. The trials randomized a total of 2,510 patients. Patients were required to have a history of 2 or more asthma exacerbations requiring oral or systemic corticosteroid treatment in the past 12 months, ACQ‑6 score of 1.5 or more at screening, and reduced lung function at baseline [pre-bronchodilator FEV1 below 80% in adults, and below 90% in adolescents] despite regular treatment with high dose ICS (SIROCCO) or with medium or high dose ICS (CALIMA) plus a long-acting beta agonist (LABA) with or without oral corticosteroids (OCS) and additional asthma controller medications. Patients were stratified by geography, age, and blood eosinophils count (≥300 cells/μL or <300 cells/μL). FASENRA administered once every 4 weeks for the first 3 doses, and then every 4 or 8 weeks thereafter as add-on to background treatment was evaluated compared to placebo.
All subjects continued their background asthma therapy throughout the duration of the trials.
ZONDA was a randomized, double-blind, parallel-group, OCS reduction trial in 220 adult patients with asthma. Patients were required treatment with daily OCS (7.5 to 40 mg per day) in addition to regular use of high-dose ICS and LABA with or without additional controller(s). The trial included an 8‑week run-in period during which the OCS was titrated to the minimum effective dose without losing asthma control. For the purposes of the OCS dose titration, asthma control was assessed by the investigator based on a patient’s FEV1, peak expiratory flow, nighttime awakenings, short-acting bronchodilator rescue medication use or any other symptoms that would require an increase in OCS dose. Baseline median OCS dose was similar across all treatment groups. Patients were required to have blood eosinophil counts greater than or equal to 150 cells/μL and a history of at least one exacerbation in the past 12 months. The baseline median OCS dose was 10 mg (range: 8 to 40 mg) for all 3 treatment groups (placebo, FASENRA every 4 weeks, and FASENRA every 4 weeks for the first 3 doses, and then once every 8 weeks).
While 2 dosing regimens were studied in SIROCCO, CALIMA, and ZONDA, the recommended dosing regimen is 30 mg FASENRA administered every 4 weeks for the first 3 doses, then every 8 weeks thereafter [see Dosage and Administration (2.1)].
| Total Population | |||
|---|---|---|---|
|
SIROCCO (N=1204) |
CALIMA (N=1306) |
ZONDA (N=220) |
|
|
Mean age (yr) |
49 |
49 |
51 |
|
Female (%) |
66 |
62 |
61 |
|
White (%) |
73 |
84 |
93 |
|
Duration of asthma, median (yr) |
15 |
16 |
12 |
|
Never smoked (%) |
80 |
78 |
79 |
|
Mean baseline FEV1 pre-bronchodilator (L) |
1.67 |
1.76 |
1.85 |
|
Mean baseline % predicted FEV1 |
57 |
58 |
60 |
|
Mean post-SABA FEV1/FVC (%) |
66 |
65 |
62 |
|
Mean baseline eosinophil count (cells/μL) |
472 |
472 |
575 |
|
Mean number of exacerbations in previous year |
3 |
3 |
3 |
Exacerbations
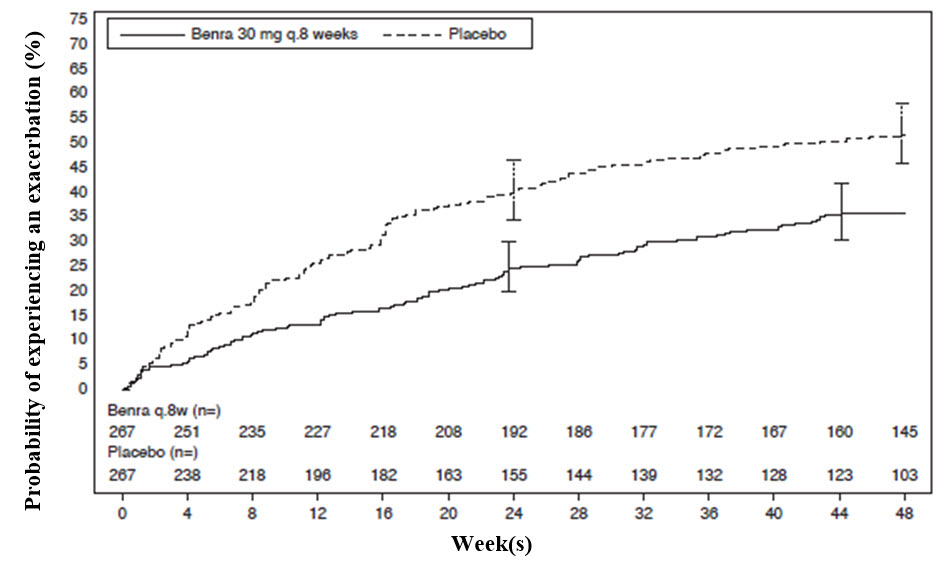
The primary endpoint for SIROCCO and CALIMA was the rate of asthma exacerbations in patients with baseline blood eosinophil counts of greater than or equal to 300 cells/μL who were taking high‑dose ICS and LABA. Asthma exacerbation was defined as a worsening of asthma requiring use of oral/systemic corticosteroids for at least 3 days, and/or emergency department visits requiring use of oral/systemic corticosteroids and/or hospitalization. For patients on maintenance oral corticosteroids, an asthma exacerbation requiring oral corticosteroids was defined as a temporary increase in stable oral/systemic corticosteroids for at least 3 days or a single depo-injectable dose of corticosteroids. In SIROCCO, 35% of patients receiving FASENRA experienced an asthma exacerbation compared to 51% on placebo. In CALIMA, 40% of patients receiving FASENRA experienced an asthma exacerbation compared to 51% on placebo (Table 4).
|
Trial |
Treatment |
Exacerbations per year |
||
|
Rate |
Difference |
Rate Ratio (95% CI) |
||
|
All exacerbations |
||||
|
SIROCCO |
FASENRA† (n=267) |
0.74 |
-0.78 |
0.49 (0.37, 0.64) |
|
Placebo (n=267) |
1.52 |
-- |
-- |
|
|
CALIMA |
FASENRA† (n=239) |
0.73 |
-0.29 |
0.72 (0.54, 0.95) |
|
Placebo (n=248) |
1.01 |
-- |
-- |
|
|
||||
|
SIROCCO |
FASENRA† (n=267) |
0.09 |
-0.16 |
0.37 (0.20, 0.67) |
|
Placebo (n=267) |
0.25 |
-- |
-- |
|
|
CALIMA |
FASENRA† (n=239) |
0.12 |
0.02 |
1.23 (0.64, 2.35) |
|
Placebo (n=248) |
0.10 |
-- |
-- |
|
|
Exacerbations requiring hospitalization |
||||
|
SIROCCO |
FASENRA† (n=267) |
0.07 |
-0.07 |
0.48 (0.22, 1.03) |
|
Placebo (n=267) |
0.14 |
-- |
-- |
|
|
CALIMA |
FASENRA† (n=239) |
0.07 |
0.02 |
1.48 (0.65, 3.37) |
|
Placebo (n=248) |
0.05 |
-- |
-- |
|
The time to first exacerbation was longer for the patients receiving FASENRA compared with placebo in SIROCCO (Figure 2). Similar findings were seen in CALIMA.
Figure 2. Kaplan-Meier Cumulative Incidence Curves for Time to First Exacerbation, SIROCCO
Subgroup analyses from SIROCCO and CALIMA identified patients with a higher prior exacerbation history and baseline blood eosinophil count as potential predictors of improved treatment response. Reductions in exacerbation rates were observed irrespective of baseline peripheral eosinophil counts; however, patients with a baseline blood eosinophil count ≥300 cells/μL showed a numerically greater response than those with counts <300 cells/μL. In both trials patients with a history of 3 or more exacerbations within the 12 months prior to FASENRA randomization showed a numerically greater exacerbation response than those with fewer prior exacerbations.
Oral Corticosteroid Reduction
ZONDA evaluated the effect of FASENRA on reducing the use of maintenance oral corticosteroids in adult patients with asthma. The primary endpoint was percent reduction from baseline of the final OCS dose during Weeks 24 to 28, while maintaining asthma control (see definition of asthma control in trial description). Compared to placebo, patients receiving FASENRA achieved greater reductions in daily maintenance oral corticosteroid dose, while maintaining asthma control. The median percent reduction in daily OCS dose from baseline was 75% in patients receiving FASENRA (95% CI: 60, 88) compared to 25% in patients receiving placebo (95% CI: 0, 33). Reductions of 50% or higher in the OCS dose were observed in 48 (66%) patients receiving FASENRA compared to those receiving placebo 28 (37%). The proportion of patients with a mean final dose less than or equal to 5 mg at Weeks 24 to 28 was 59% for FASENRA and 33% for placebo (odds ratio 2.74, 95% CI: 1.41, 5.31). Only patients with an optimized baseline OCS dose of 12.5 mg or less were eligible to achieve a 100% reduction in OCS dose during the study. Of those patients, 52% (22 of 42) receiving FASENRA and 19% (8 of 42) on placebo achieved a 100% reduction in OCS dose. Exacerbations resulting in hospitalization and/or ER visit were also assessed as a secondary endpoint. In this 28-week trial, patients receiving FASENRA had 1 event while those on placebo had 14 events (annualized rate 0.02 and 0.32, respectively; rate ratio of 0.07, 95% CI: 0.01, 0.63).
Lung Function
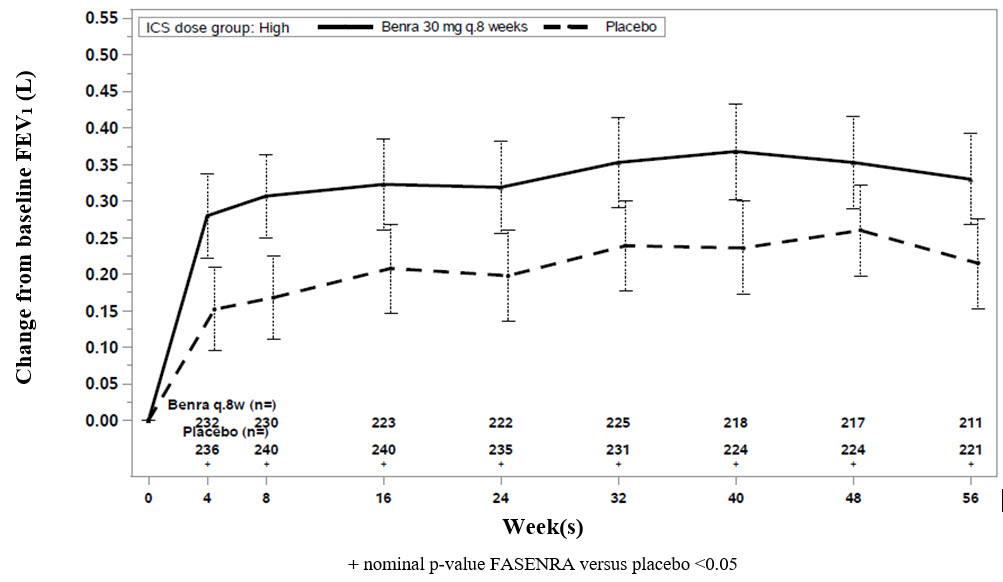
Change from baseline in mean FEV1 was assessed in SIROCCO, CALIMA, and ZONDA as a secondary endpoint. Compared with placebo, FASENRA provided consistent improvements over time in the mean change from baseline in FEV1 (Figure 3 and Table 5).
Figure 3. Mean Change from Baseline in Pre-Bronchodilator FEV1 (L), CALIMA
|
|
|
Trial |
Difference from Placebo in Mean Change from Pre-Bronchodilator Baseline FEV1 (L) (95% CI) |
|
SIROCCO |
0.159 (0.068, 0.249) |
|
CALIMA |
0.116 (0.028, 0.204) |
|
ZONDA |
0.112 (-0.033, 0.258) |
Subgroup analyses also showed greater improvements in FEV1 in patients with higher baseline blood eosinophil counts and more frequent prior exacerbation history.
The clinical program for FASENRA also included a 12-week, randomized, double-blind, placebo-controlled lung function trial conducted in 211 adult patients with mild to moderate asthma. Patients were treated with placebo or benralizumab 30 mg SC every 4 weeks for 3 doses. Lung function, as measured by the change from baseline in FEV1 at Week 12 was improved in the benralizumab treatment group compared to placebo.
Patient Reported Outcomes
The Asthma Control Questionnaire-6 (ACQ-6) and Standardized Asthma Quality of Life Questionnaire for 12 Years and Older (AQLQ(S)+12) were assessed in SIROCCO, CALIMA, and ZONDA. The responder rate for both measures was defined as improvement in score of 0.5 or more as threshold at the end of SIROCCO, CALIMA, and ZONDA (48, 56, and 28 weeks, respectively). In SIROCCO, the ACQ-6 responder rate for FASENRA was 60% vs 50% placebo (odds ratio 1.55; 95% CI: 1.09, 2.19). In CALIMA, the ACQ-6 responder rate for FASENRA was 63% vs 59% placebo (odds ratio 1.16; 95% CI: 0.80, 1.68). In SIROCCO, the responder rate for AQLQ(S)+12 for FASENRA was 57% vs 49% placebo (odds ratio 1.42; 95% CI: 0.99, 2.02), and in CALIMA, 60% FASENRA vs 59% placebo (odds ratio of 1.03; 95% CI: 0.70,1.51). Similar results were seen in ZONDA.
14.2 Clinical Studies in Patients with Eosinophilic Granulomatosis with Polyangiitis
The efficacy of FASENRA for eosinophilic granulomatosis with polyangiitis (EGPA) was evaluated in a randomized, double-blind, active-controlled, noninferiority clinical trial (MANDARA [NCT04157348]) of 52‑weeks duration. The trial enrolled a total of 140 adults aged 18 years and older with EGPA. Patients were required to have asthma, eosinophilia (1,000 cells/uL or >10% of leukocytes) and a history of relapsing or refractory disease treated with background prednisolone/prednisone with or without immunosuppressive therapy. Patients were randomized to receive FASENRA 30 mg administered subcutaneously every 4 weeks or mepolizumab 300 mg administered subcutaneously every 4 weeks in addition to continued background therapy. Starting at Week 4, the oral corticosteroid (OCS) dose was tapered at the discretion of the investigator. The MANDARA trial was a non-inferiority trial and was not designed to assess whether FASENRA was superior to mepolizumab. The pre-specified noninferiority (NI) margin was a treatment difference of ‑25%. The secondary endpoints (accrued duration of remission, relapse, OCS reduction and the asthma control questionnaire-6) were not included in the pre-specified multiple testing procedure for statistical significance.
The demographics and baseline characteristics of patients in MANDARA are provided in Table 6.
| MANDARA (N=140) | |
|---|---|
|
Mean age (years) |
52 |
|
Female (%) |
60 |
|
White (%) |
79 |
|
Asian (%) |
12 |
|
Other (%) |
4 |
|
Hispanic or Latino (%) |
3 |
|
Time since diagnosis of EGPA, years, mean (SD) |
5.2 (5.6) |
|
History of ≥1 confirmed relapse in past 2 years (%) |
79 |
|
Refractory disease (%) |
60 |
|
Baseline oral corticosteroid* daily dose, mg, median (range) |
10 (5 - 40) |
|
Baseline BVAS, median (range) |
0 (0 – 18) |
|
BVAS=0 (%) |
52 |
|
Receiving immunosuppressive therapy† (%) |
36 |
|
ANCA positive‡ (%) |
29 |
|
Biopsy Evidence of Eosinophilic Vasculitis/Inflammation (%) |
38 |
|
SD=standard deviation; BVAS=Birmingham vasculitis activity score. |
|
Remission
The primary endpoint in MANDARA was the proportion of patients in remission, defined as Birmingham Vasculitis Activity Score (BVAS)=0 (no active vasculitis) plus prednisolone/prednisone dose ≤4 mg/day, at both Week 36 and Week 48. The BVAS is a clinician-completed tool, that is divided into 9 organ-based systems, to assess clinically active vasculitis that would likely require treatment, after exclusion of other causes. As shown in Table 7, FASENRA demonstrated noninferiority to mepolizumab for the primary endpoint of remission and the components of remission.
|
||||||
|
Remission
(OCS≤4 mg/day + |
OCS≤4 mg/day |
BVAS=0 |
||||
|
FASENRA* N=70 |
Mepo† N=70 |
FASENRA* N=70 |
Mepo† N=70 |
FASENRA* N=70 |
Mepo† N=70 |
|
|
Patients in remission at both Weeks 36 and 48 |
||||||
|
Patients, n (%)‡ |
41 (59) |
40 (57) |
43 (62) |
41 (58) |
58 (83) |
59 (84) |
|
Differences in remission rate (%)‡ (95% CI) |
2.7 |
-- |
4.1 |
-- |
-1.2 |
-- |
|
(-13, 18)§ |
-- |
(-11, 19) |
-- |
(-13, 11) |
-- |
|
|
N=number of patients in analysis. |
||||||
Using an alternative remission definition of BVAS=0 plus prednisolone/ prednisone ≤7.5 mg/day, consistent efficacy between groups for these endpoints was observed.
Accrued Duration of Remission
Total accrued duration of remission was similar in FASENRA compared to mepolizumab (odds ratio 1.4, 95% CI: 0.75, 2.5). Results for accrued duration of remission are shown in Table 8. The proportion of patients achieving remission within the first 24 weeks of treatment and remaining in remission through Week 52 was 42% for FASENRA and 37% for mepolizumab (difference in responder rate 5.5%, 95% CI: -9.3, 20). This result was not statistically significant as there was no pre-specified multiple testing procedure.
|
||||||
|
Remission (OCS≤4 mg/day + BVAS=0) |
OCS≤4 mg/day |
BVAS=0 |
||||
|
FASENRA* N=70 |
Mepo† N=70 |
FASENRA* N=70 |
Mepo† N=70 |
FASENRA* N=70 |
Mepo† N=70 |
|
|
Accrued duration over 52 weeks‡, n (%) |
||||||
|
0 weeks§ >0 to <12 weeks 12 to <24 weeks 24 to <36 weeks ≥36 weeks |
9 (13) 12 (17) 8 (11) 21 (30) 20 (29) |
15 (21) 10 (14) 8 (11) 19 (27) 18 (26) |
9 (13) 10 (14) 9 (13) 19 (27) 23 (33) |
12 (17) 12 (17) 8 (11) 18 (26) 20 (29) |
0 0 2 (3) 6 (9) 62 (89) |
0 2 (3) 2 (3) 7 (10) 59 (84) |
|
Odds ratio¶
|
1.4 (0.75, 2.5) |
-- -- |
1.4 (0.74, 2.5) |
-- -- |
1.5 (0.54, 4.2) |
-- -- |
Relapse
The hazard ratio for time to first relapse (defined as worsening related to vasculitis, asthma, or sino-nasal symptoms requiring an increase in dose of corticosteroids or immunosuppressive therapy or hospitalization) was 0.98 (95% CI: 0.53, 1.8). Relapse was observed in 30% of patients on FASENRA and 30% of patients on mepolizumab. The annualized relapse rate was 0.50 for patients receiving FASENRA versus 0.49 for patients receiving mepolizumab (rate ratio 1.0, 95% CI: 0.56, 1.9). The types of relapse were consistent for patients receiving FASENRA or mepolizumab.
Oral Corticosteroid Reduction
During Weeks 48 to 52, a 100% reduction in the OCS dose was observed in 41% of patients receiving FASENRA compared to 26% of those receiving mepolizumab (difference 16%, 95% CI: 0.67, 31). During Weeks 48 to 52, reductions of 50% or higher were observed in 86% of patients receiving FASENRA compared to 74% of those receiving mepolizumab (difference 12%, 95% CI: -0.57, 25). These results were not statistically significant as there was no pre‑specified multiple testing procedure.
Asthma Control Questionnaire-6 (ACQ-6)
ACQ-6 is a 6-item questionnaire that is completed by the patient to measure the adequacy of asthma control and change in asthma control. The ACQ-6 responder rate during Weeks 48 to 52 (defined as a decrease in score of 0.5 or more compared with baseline) was 42% for FASENRA and 48% for mepolizumab (difference -6.2%, 95% CI: -19, 6.2).
16. How is Fasenra supplied
How Supplied
FASENRA (benralizumab) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution and may contain a few translucent or white to off-white particles, for subcutaneous injection supplied as a single-dose prefilled syringe or single-dose autoinjector. The prefilled syringe (including stopper and cap) and autoinjector are not made with natural rubber latex.
FASENRA is available as:
Single-Dose Prefilled Syringe
- •
- Carton contains one 10 mg/0.5 mL single-dose prefilled syringe with a gray plunger rod: NDC 0310-1745-01
- •
- Carton contains one 30 mg/mL single-dose prefilled syringe with a blue plunger rod: NDC 0310-1730-30
Single-Dose Autoinjector FASENRA PEN
- •
- Carton contains one 30 mg/mL single-dose autoinjector: NDC 0310-1830-30
Storage and Handling
Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
If needed, the prefilled syringe and autoinjector may be stored at room temperature up to 77°F (25°C) for a maximum of 14 days in the original carton to protect from light. Once removed from the refrigerator and brought to room temperature (up to 77°F [25°C]), the prefilled syringe and autoinjector must be used within 14 days or discarded.
Do not freeze. Do not shake. Do not expose to heat.
17. Patient Counseling Information
Advise the patients and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use for FASENRA PEN) before the patient starts using FASENRA and each time the prescription is renewed as there may be new information they need to know.
Administration Instructions
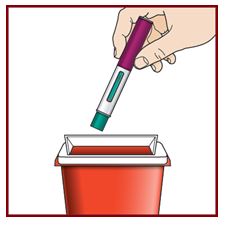
Instruct patients and/or caregivers on proper subcutaneous injection technique using the FASENRA PEN, including aseptic technique, and the preparation and administration of FASENRA PEN prior to use. Advise patients to follow sharps disposal recommendations [see Instructions for Use].
Hypersensitivity Reactions
Inform patients that hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, rash) have occurred after administration of FASENRA. These reactions generally occurred within hours of FASENRA administration, but in some instances had a delayed onset (i.e., days). Instruct patients to contact their healthcare provider if they experience symptoms of an allergic reaction [see Warnings and Precautions (5.1)].
Not for Acute Symptoms or Deteriorating Disease
Inform patients that FASENRA does not treat acute asthma symptoms or acute exacerbations. Inform patients to seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with FASENRA [see Warnings and Precautions (5.2)].
Reduction of Corticosteroid Dosage
Inform patients to not discontinue systemic or inhaled corticosteroids except under the direct supervision of a physician. Inform patients that reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy [see Warnings and Precautions (5.3)].
Manufactured by
AstraZeneca AB
Södertälje, Sweden SE-15185
US License No. 2059
Distributed by
AstraZeneca Pharmaceuticals LP,
Wilmington, DE 19850
FASENRA is a trademark of the AstraZeneca group of companies.
©AstraZeneca 2024
|
Patient Information FASENRA® (fas-en-rah) (benralizumab) injection, for subcutaneous use |
|
What is FASENRA? FASENRA is a prescription medicine used:
It is not known if FASENRA is safe and effective in children with asthma under 6 years of age. It is not known if FASENRA is safe and effective in children with EGPA under 18 years of age. |
|
Do not use FASENRA if you are allergic to benralizumab or any of the ingredients in FASENRA. See the end of this leaflet for a complete list of ingredients in FASENRA. |
|
Before using FASENRA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Do not stop taking your other medicines for your condition unless your healthcare provider tells you to. |
|
How will I use FASENRA?
|
|
What are the possible side effects of FASENRA? FASENRA may cause serious side effects, including:
The most common side effects of FASENRA include headache and sore throat. These are not all the possible side effects of FASENRA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store FASENRA?
|
|
General information about the safe and effective use of FASENRA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use FASENRA for a condition for which it was not prescribed. Do not give FASENRA to other people, even if they have the same symptoms you have. It may harm them. You can ask your doctor or pharmacist for information about FASENRA that is written for healthcare providers. |
|
What are the ingredients in FASENRA? Active ingredient: benralizumab Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 20, α,α-trehalose dihydrate, and Water for Injection Manufactured by: AstraZeneca AB, Södertälje, Sweden SE-15185 U.S. License Number 2059 Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850 FASENRA is a trademark of the AstraZeneca group of companies. ©AstraZeneca 2024 For more information, go to https://www.FASENRA.com or call 1-800-236-9933. |
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: September 2024
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0310-1730-30 Rx only
|
FASENRA® (benralizumab) Injection For Subcutaneous Injection Only Store the prefilled syringe refrigerated at 36°F - 46°F (2°C - 8°C) in original carton to protect from light. Do not shake, freeze, or expose to heat. |
30 mg/mL 1 single-dose prefilled syringe Discard unused portion. AstraZeneca |
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0310-1745-01 Rx only
|
FASENRA® (benralizumab) Injection For Subcutaneous Injection Only Store the prefilled syringe refrigerated at 36°F - 46°F (2°C - 8°C) in original carton to protect from light. Do not shake, freeze, or expose to heat. |
10 mg/0.5 mL 1 single-dose prefilled syringe Discard unused portion. AstraZeneca |
Package/Label Display Panel
NDC 0310-1830-30 Rx only
|
FASENRA PEN™ (benralizumab) Injection For Subcutaneous Injection Only Store the FASENRA PEN refrigerated at 36°F - 46°F (2°C - 8°C) in original carton to protect from light. Do not shake, freeze, or expose to heat. |
30 mg/mL 1 single-dose prefilled autoinjector Discard unused portion. AstraZeneca |
| FASENRA
benralizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| FASENRA
benralizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| FASENRA
benralizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - AstraZeneca Pharmaceuticals LP (054743190) |
| Registrant - AstraZeneca PLC (230790719) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AstraZeneca AB | 631892705 | MANUFACTURE(0310-1730, 0310-1830, 0310-1745) | |
Biological Products Related to Fasenra
Find detailed information on biosimilars for this medication.
Frequently asked questions
- What are monoclonal antibodies?
- Does Fasenra weaken your immune system?
- How long does Fasenra take to work?
- What is Fasenra used for and how does it work?
- What eosinophil count is needed for Fasenra?
- What type of asthma is Fasenra used to treat?
More about Fasenra (benralizumab)
- Check interactions
- Compare alternatives
- Reviews (76)
- Drug images
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- FDA approval history
- Drug class: interleukin inhibitors
- Breastfeeding