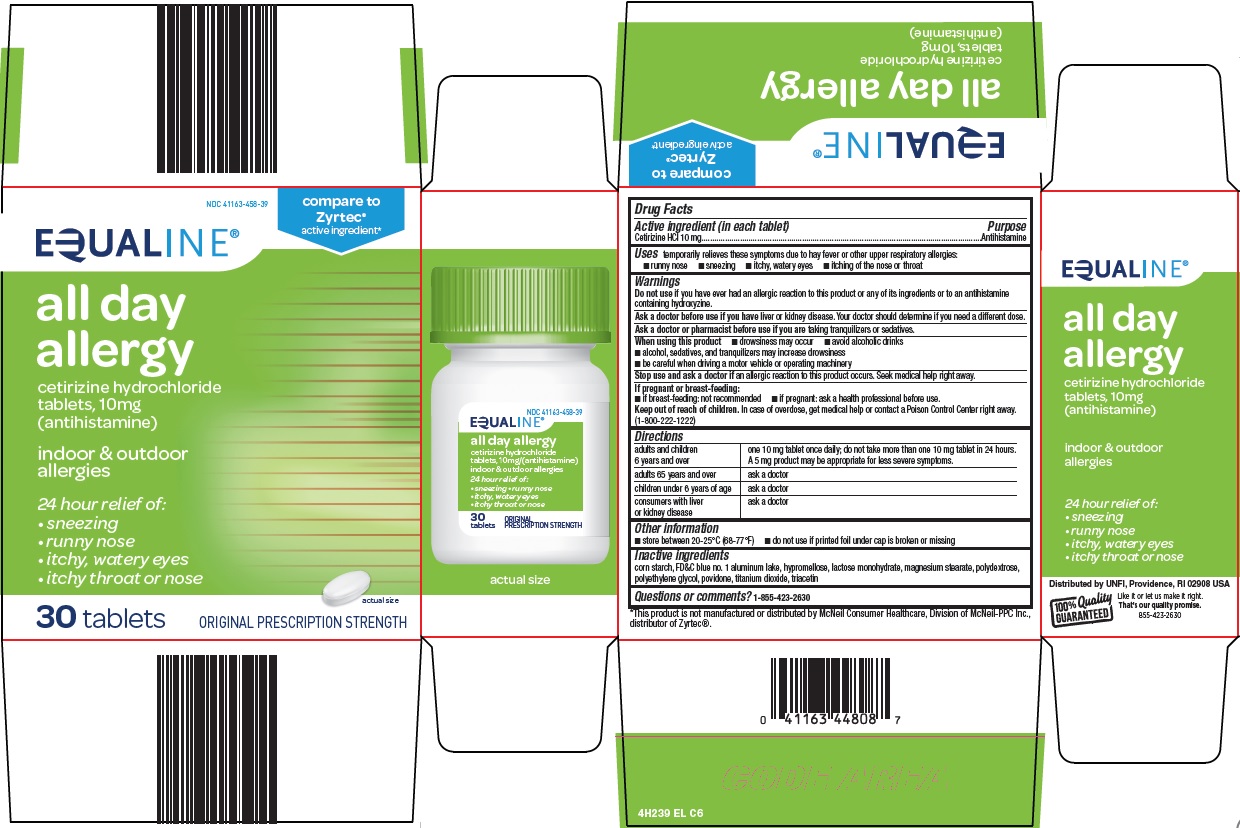
Equaline All Day Allergy: Package Insert / Prescribing Info
Package insert / product label
Generic name: cetirizine hydrochloride
Dosage form: tablet
Drug class: Antihistamines
Medically reviewed by Drugs.com. Last updated on Jul 25, 2025.
Indications and Usage for Equaline All Day Allergy
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Equaline All Day Allergy Dosage and Administration
|
adults and children 6 years and over |
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. |
|
adults 65 years and over |
ask a doctor |
|
children under 6 years of age |
ask a doctor |
|
consumers with liver or kidney disease |
ask a doctor |
Related/similar drugs
Storage and Handling
- •
- store between 20 - 25°C (68 - 77°F)
- •
- do not use if printed foil under cap is broken or missing
Inactive ingredients
corn starch, FD&C blue no. 1 aluminum lake, hypromellose, lactose monohydrate, magnesium stearate, polydextrose, polyethylene glycol, povidone, titanium dioxide, triacetin
Principal Display Panel
compare to Zyrtec® active ingredient
EQUALINE®
all day allergy
cetirizine hydrochloride tablets, 10mg (antihistamine)
indoor & outdoor allergies
24 hour relief of:
sneezing
runny nose
itchy, watery eyes
itchy throat or nose
actual size
30 tablets
ORIGINAL PRESCRIPTION STRENGTH
| EQUALINE ALL DAY ALLERGY
cetirizine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - United Natural Foods, Inc. dba UNFI (943556183) |
Frequently asked questions
- Is it OK to take antihistamines every day?
- Should cetirizine be taken at bedtime or upon awakening?
- Which antihistamines make you drowsy?
- Can you take antihistamines when pregnant?
- Is Generic Zyrtec Available?
More about All Day Allergy (cetirizine)
- Check interactions
- Compare alternatives
- Reviews (1)
- Imprints, shape & color data
- Latest FDA alerts (3)
- Side effects
- Dosage information
- During pregnancy
- Drug class: antihistamines
- Breastfeeding
- En español
Professional resources
Other brands
Zyrtec, Aller-Tec, Quzyttir, Zyrtec Hives, Aller-Tec Children's