Encelto: Package Insert / Prescribing Info
Package insert / product label
Generic name: revakinagene taroretcel-lwey
Dosage form: intravitreal implant
Medically reviewed by Drugs.com. Last updated on Mar 30, 2025.
On This Page
Highlights of Prescribing Information
These highlights do not include all the information needed to use ENCELTO™ safely and effectively. See full prescribing information for ENCELTO.
ENCELTO (revakinagene taroretcel-lwey) implant, for intravitreal use
Initial U.S. Approval: 2025
Indications and Usage for Encelto
ENCELTO is an allogeneic encapsulated cell-based gene therapy indicated for the treatment of adults with idiopathic macular telangiectasia type 2 (MacTel). (1)
Encelto Dosage and Administration
For intravitreal implantation only.
- ENCELTO is intended for surgical intravitreal implantation under aseptic conditions by a qualified ophthalmologist. (2.1)
- The recommended dose is one ENCELTO implant per affected eye containing 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF). (2.1)
- Carefully inspect ENCELTO prior to use and refer to the Instructions for Use when preparing for and performing surgical placement or removal of ENCELTO. (2.2, 2.3)
Dosage Forms and Strengths
One single-dose implant containing 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing rhCNTF. (3)
Contraindications
Warnings and Precautions
- ENCELTO implantation has been associated with severe vision loss, infectious endophthalmitis, retinal tears and/or detachment, vitreous hemorrhage, implant extrusion, cataract formation, suture related complications, and delayed dark adaptation. Patients should be instructed to report signs or symptoms that could be associated with these events without delay. Additional surgical and/or medical management may be required. (5.1, 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8)
- Vitreous Hemorrhage: Temporarily discontinue antithrombotic medication prior to ENCELTO insertion surgery to reduce the risk of implantation related vitreous hemorrhage. Vitreous hemorrhages occurring greater than one year from implantation could be a sign of ENCELTO extrusion. The surgical site should be examined closely and the ENCELTO should be surgically repositioned if indicated. (5.4)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥2%) were conjunctival hemorrhage, delayed dark adaptation, foreign body sensation, eye pain, suture related complications, miosis, conjunctival hyperemia, eye pruritus, ocular discomfort, vitreous hemorrhage, blurred vision, headache, dry eye, eye irritation, cataract progression or formation, vitreous floaters, severe vision loss, eye discharge, anterior chamber cell, iridocyclitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Neurotech at 1-833-963-9275 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for FDA-approved patient labeling.
Revised: 3/2025
Full Prescribing Information
1. Indications and Usage for Encelto
ENCELTO is indicated for the treatment of adults with idiopathic macular telangiectasia type 2 (MacTel).
2. Encelto Dosage and Administration
2.1 Recommended Dose
For intravitreal implantation only
• ENCELTO is administered by a single surgical intravitreal procedure performed by a qualified ophthalmologist.
• The recommended dose is one ENCELTO implant per affected eye. Each ENCELTO implant contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF) (NTC-201-6A cell line), a neurotrophic factor.
2.2 ENCELTO Surgical Placement
The ENCELTO implant insertion is a surgical procedure performed in an operating room under aseptic conditions by a qualified ophthalmologist.
|
Pre-Surgical Preparation
Prepare the surgical field properly. |
|
|
Surgical Steps |
|
|
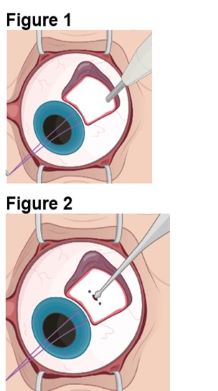
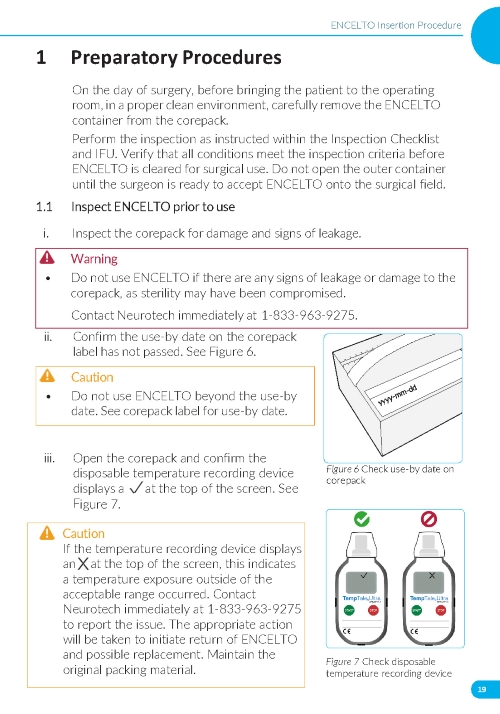
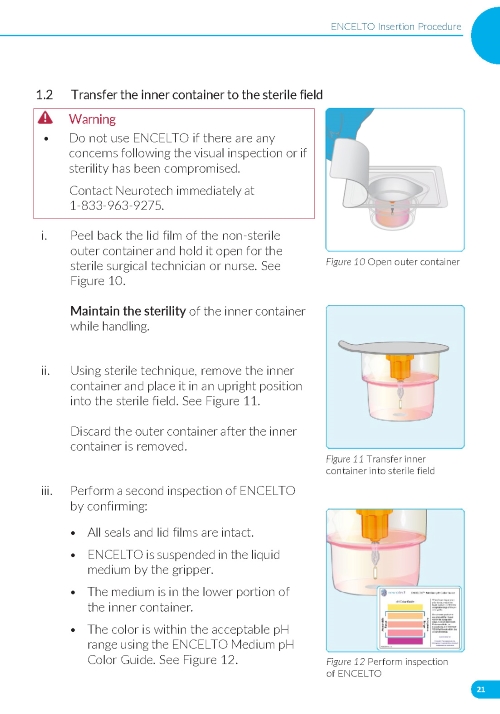
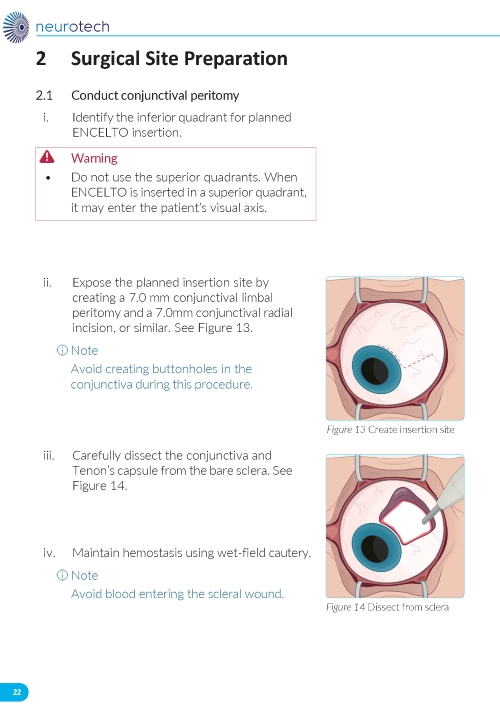
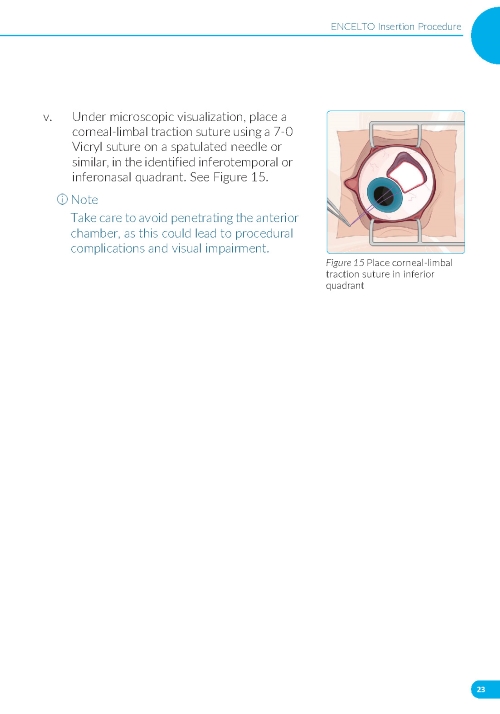
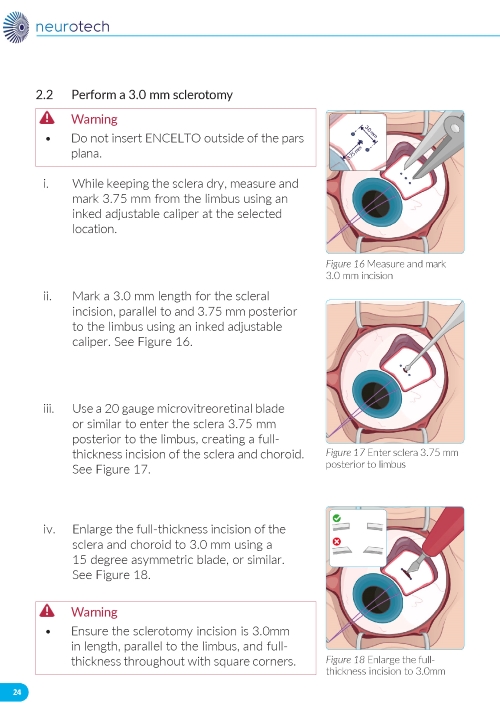
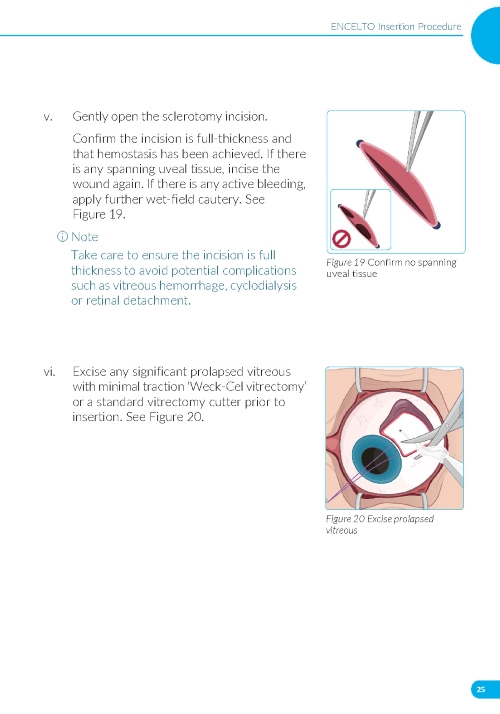
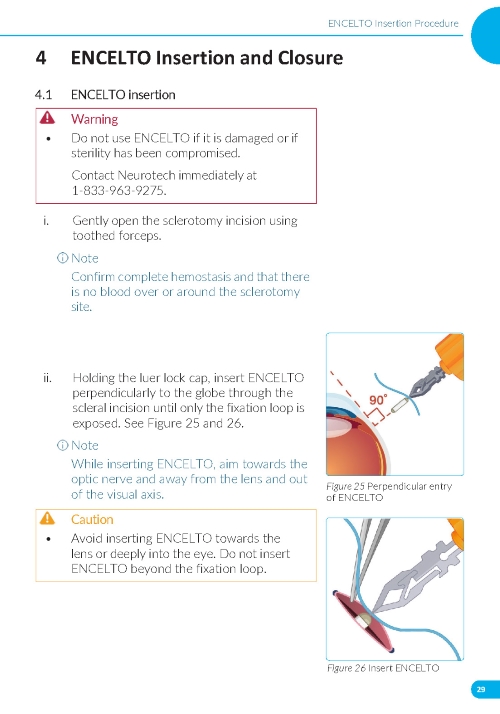
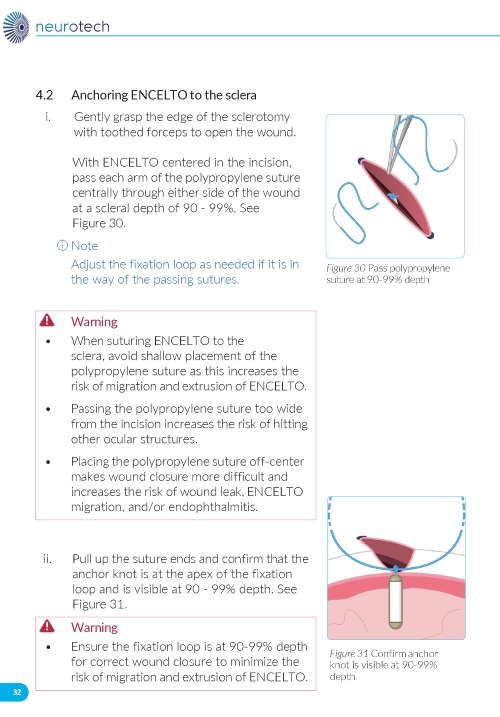
1. Preparing the Surgical Site a. Create a 7x7 mm peritomy of the conjunctiva and Tenon’s capsule at the selected implantation site. b. Place a corneal-limbal traction suture in the selected surgical quadrant (either inferotemporal or inferonasal) (Figure 1). c. Maintain hemostasis of the underlying sclera and conjunctiva (Figure 1). d. Using an MVR and 15-degree blade, create a 3.0 mm full-thickness sclerotomy 3.75 mm posterior and parallel to the limbus (Figure 2). Do not insert ENCELTO outside of the pars plana. e. Confirm: • The incision is full thickness. • There is adequate hemostasis. • There is no spanning uveal tissue. | |
|
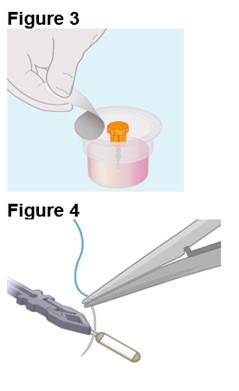
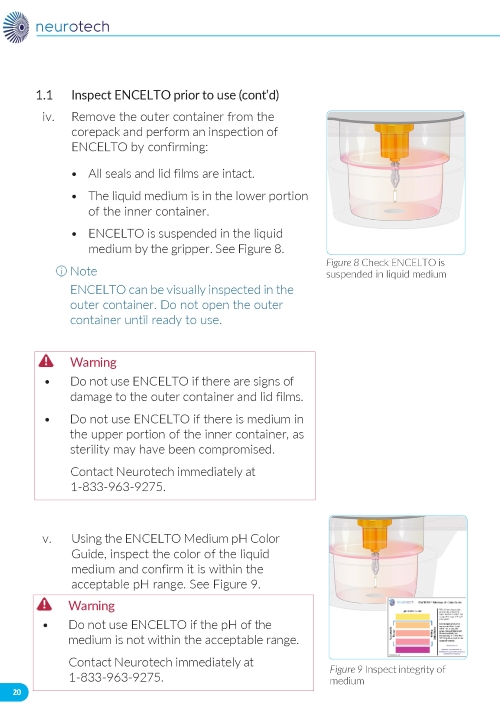
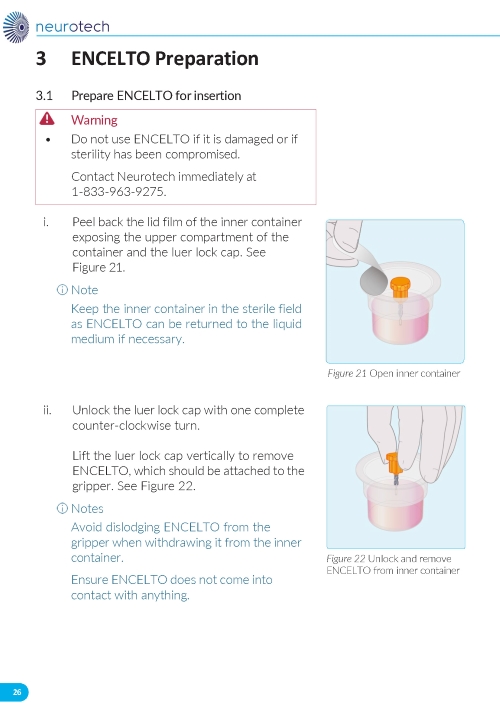
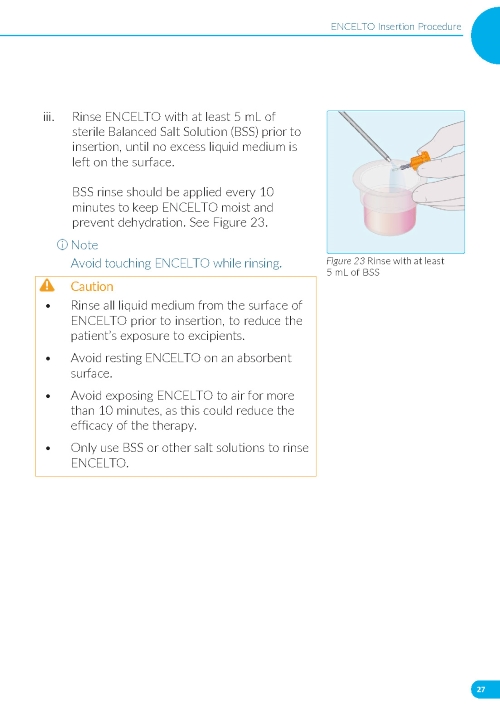
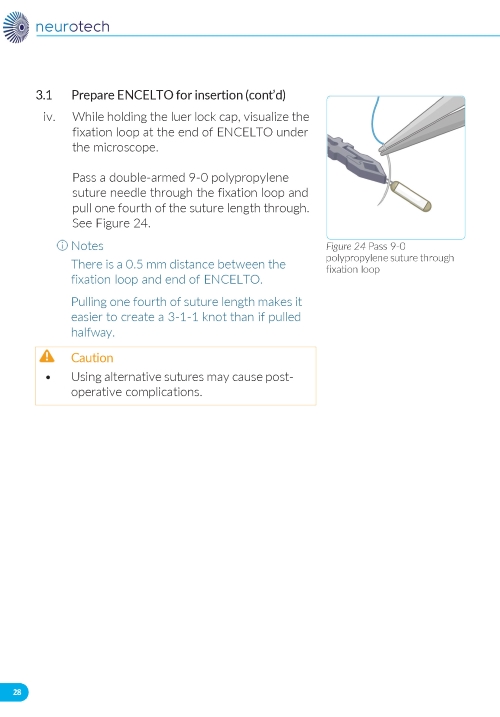
2. Preparing the ENCELTO Implant a. Open the inner container and expose the upper compartment and luer lock cap (Figure 3). b. Unlock the luer lock cap by turning it counterclockwise once. c. Lift the luer lock cap vertically to remove ENCELTO (attached to the gripper). d. Rinse ENCELTO with at least 5 mL of sterile Balanced Saline Solution (BSS). e. Keep ENCELTO moist by applying BSS every 10 minutes until insertion. f. While holding the luer lock cap, pass a double-armed 9-0 polypropylene suture needle through ENCELTO’s fixation loop (Figure 4). | |
|
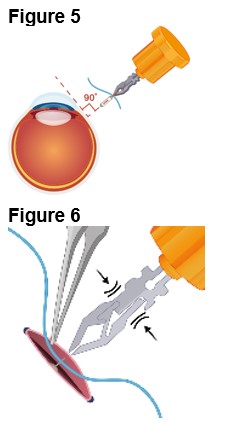
3. Implantation of ENCELTO a. Gently open the sclerotomy incision and insert ENCELTO perpendicularly into the eye (Figure 5). b. Ensure only the fixation loop is exposed. c. Release ENCELTO from the gripper by squeezing the indicated region with forceps or a fine needle holder (Figure 6). | |
|
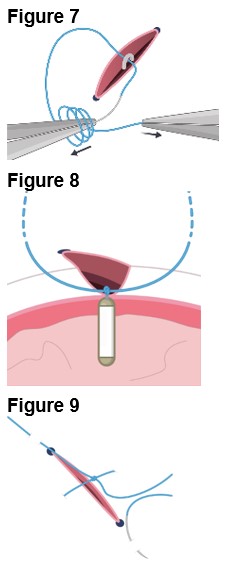
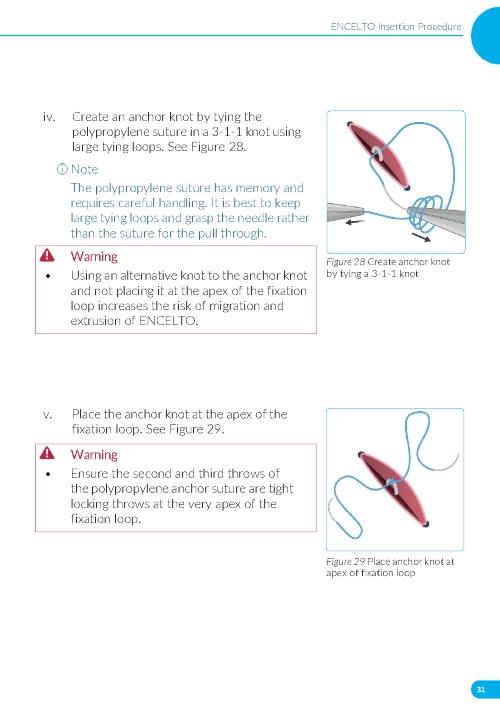
4. Securing the Implant a. Secure ENCELTO by creating a 3-1-1 anchor knot with the polypropylene suture at the apex of the fixation loop (Figure 7). b. Confirm ENCELTO is centered in the incision. c. Pass each suture arm centrally through either side of the wound at 90-99% scleral depth (Figure 8). d. Pull up the suture ends and confirm that the fixation loop is at the proper depth (90-99%). e. Tie down the suture to the sclera with a 3-1-1 knot, ensuring the knot is placed away from the incision. f. If a suture breaks, leave the tail as long as possible and lay it flat. g. Take a 2.0 mm scleral bite at 50-75% depth beyond the sclerotomy on each side (Figure 9). | |
|
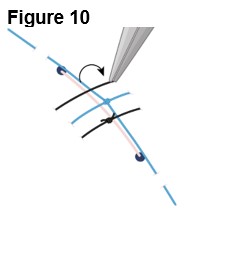
5. Closing the Incision a. Close the scleral incision with 9-0 nylon sutures (Figure 10), ensuring:
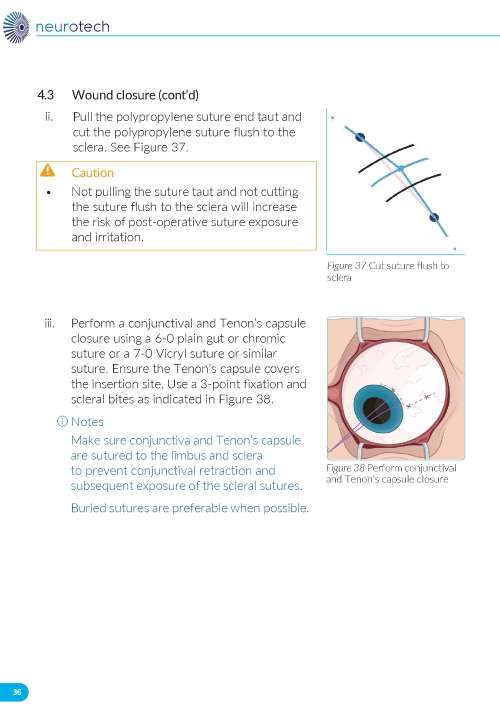
b. Pull the polypropylene suture end taut and cut it flush to the sclera. c. Close the conjunctiva and Tenon’s capsule using 6-0 plain gut or chromic suture, or 7-0 Vicryl suture or similar. d. Ensure Tenon’s capsule covers the insertion site and use 3-point fixation and scleral bites. e. Administer sub-conjunctival steroid injection: dexamethasone, 2 mg/0.5 ml (4 mg/ml) or equivalent. If the case is complicated and inflammation is anticipated, a higher dose of dexamethasone (0.5 cc of 10 mg/ml) or equivalent may be used, at the surgeon’s discretion. f. Perform indirect ophthalmoscopy to confirm placement of ENCELTO in the vitreous and that there are no intraocular complications. Failure to perform indirect ophthalmoscopy can lead to unidentified malpositioning of ENCELTO and intraocular complications. | |
|
Post-Operative Wound Care
|
|
Refer to ENCELTO Instructions for Use for detailed guidance on implantation procedure.
2.3 ENCELTO Removal Procedure
Removal of ENCELTO is a surgical procedure performed in an operating room under aseptic conditions by a qualified ophthalmologist. Remove ENCELTO implant, if vitrectomy with a complete gas fill or silicone oil fill is required or if infectious endophthalmitis occurs.
|
Surgical Steps |
|
|
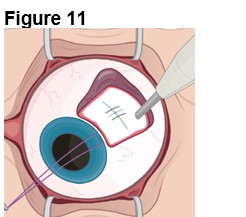
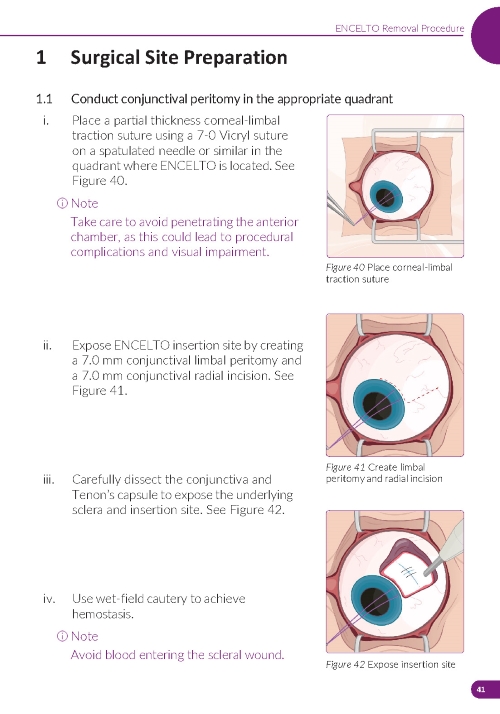
1. Preparing the Surgical Site (Figure 11) a. Create a 7x7 mm peritomy of the conjunctiva and Tenon’s capsule to expose the insertion site. b. Place a corneal-limbal traction suture in the quadrant where ENCELTO is located. c. Maintain hemostasis of the sclera and surrounding conjunctiva. | |
|
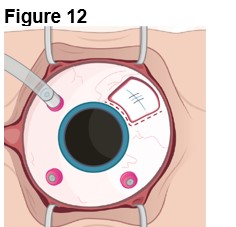
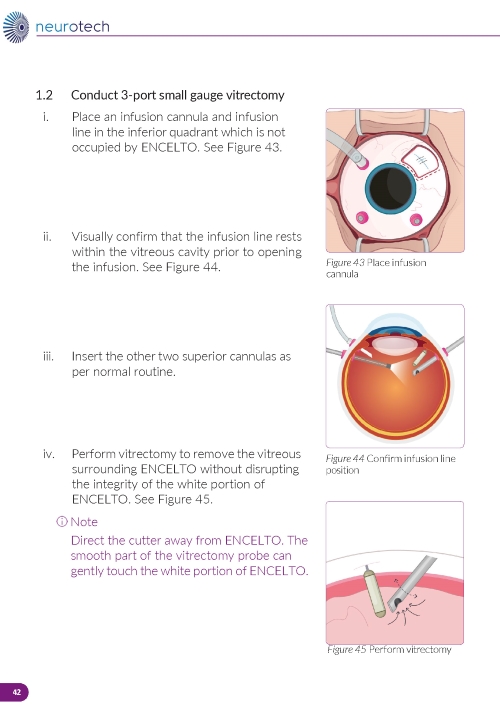
2. Establishing Infusion & Vitrectomy (Figure 12) a. Place an infusion cannula in the inferior quadrant (opposite ENCELTO). b. Confirm the infusion line is positioned within the vitreous cavity before opening the infusion. c. Insert two superior cannulas following normal pars plana vitrectomy protocol. d. Perform a thorough vitrectomy to remove vitreous surrounding ENCELTO without disrupting the hollow fiber membrane. | |
|
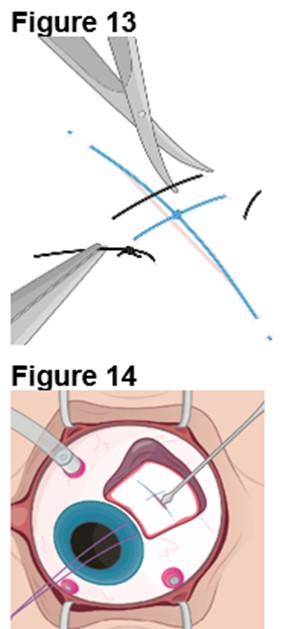
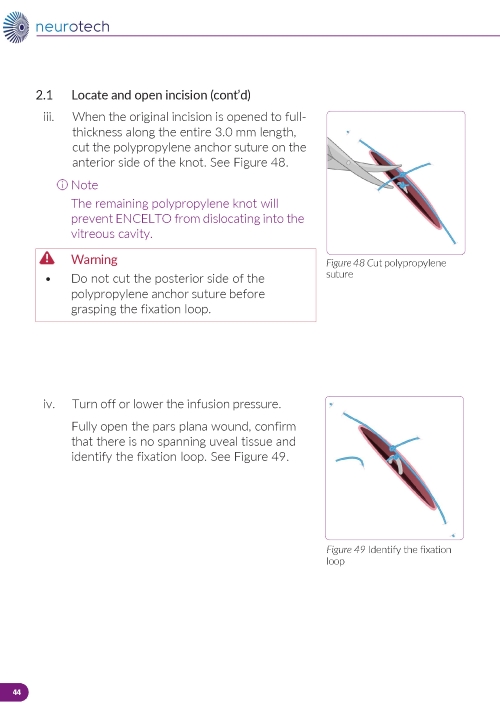
3. Reopening the Sclerotomy a. Locate the ENCELTO incision and remove the two nylon sutures while leaving the polypropylene suture intact (Figure 13). b. Using an MVR blade, carefully dissect open the original scleral incision down to the ENCELTO cap at the base of the fixation loop (Figure 14). c. Extend the incision along the entire 3.0 mm length to full thickness. d. Cut the polypropylene anchor suture on the anterior side of the knot. e. Turn off or lower infusion pressure. | |
|
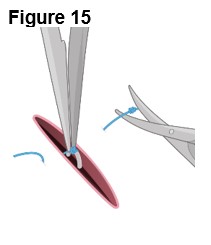
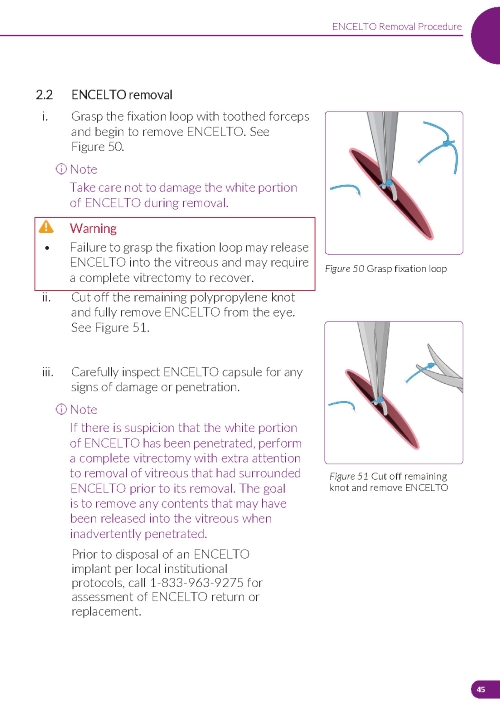
4. Removing ENCELTO (Figure 15) a. Fully open the pars plana sclerotomy and confirm there is no spanning uveal tissue. b. Identify and grasp the fixation loop. c. Cut off the remaining polypropylene knot. d. Remove ENCELTO from the eye. e. Inspect the ENCELTO capsule for any damage or penetration. f. Do not discard or dispose of the ENCELTO implant. Call and report to 1-833-963-9275. The appropriate action will be taken to initiate the return of ENCELTO and possible replacement. | |
|
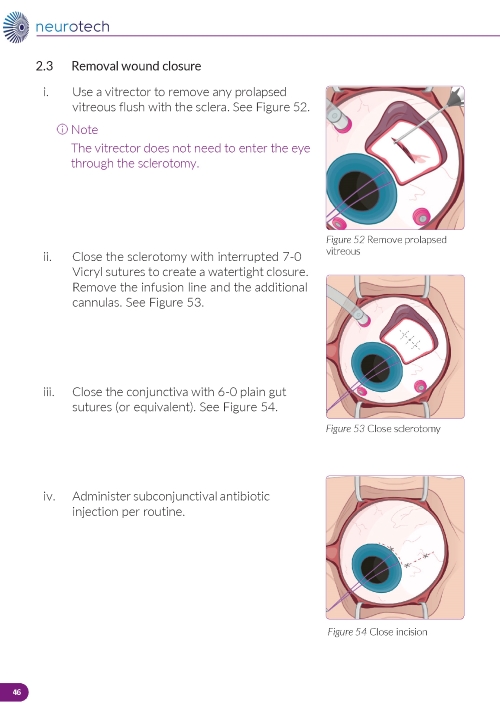
5. Closing the Incision a. Remove any prolapsed vitreous. b. Close the sclerotomy with interrupted 7-0 Vicryl sutures for a watertight closure. c. Remove the infusion line and additional cannulas. d. Close the conjunctiva with 6-0 plain gut sutures or equivalent. |
|
|
Post-Operative Wound Care
|
|
Refer to ENCELTO Instructions for Use for detailed guidance on removal procedure.
3. Dosage Forms and Strengths
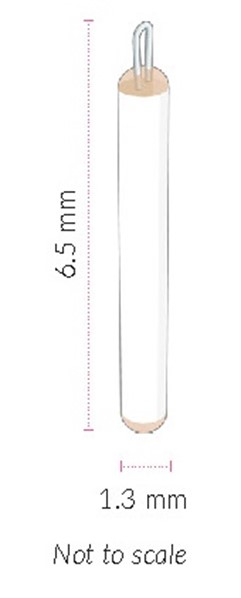
ENCELTO is a single-dose implant that contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF) (NTC-201-6A cell line) for intravitreal surgical placement. ENCELTO is an opaque semi-permeable capsule that is white to off-white, capped on both ends, and has a titanium loop on one end. The ENCELTO width is 1.2 ± 0.1 mm, its length is 6.1 ± 0.4 mm, and its internal diameter is 0.88 ± 0.02 mm (Figure 17).
4. Contraindications
ENCELTO is contraindicated in patients with:
- Active or suspected ocular or periocular infections.
- Known hypersensitivity to Endothelial Serum Free Media (Endo-SFM)
5. Warnings and Precautions
5.1 Severe Vision Loss
Severe vision loss defined as three or more lines of visual acuity loss [≥15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters] has occurred following ENCELTO implantation [see Adverse Reactions (6)]. Monitor patients for signs and symptoms of vision loss and manage as clinically indicated.
5.2 Infectious Endophthalmitis
Infectious endophthalmitis may occur following ENCELTO implantation. Signs and symptoms of infectious endophthalmitis include progressively worsening eye pain, vision loss, or scleral and conjunctival injection. To mitigate the risk of endophthalmitis, use proper aseptic surgical technique for ENCELTO implantation [see Dosage and Administration (2.2)]. Monitor patients for signs or symptoms of infectious endophthalmitis. Remove ENCELTO implant if infectious endophthalmitis occurs and manage symptoms according to clinical practice.
5.3 Retinal Tear and Detachment
Retinal tears and retinal detachment may occur following ENCELTO implantation. Signs and symptoms of retinal tears include acute onset of flashing lights, floaters, and/or loss of visual acuity. Signs and symptoms of retinal detachment may include progressive visual field loss and/or loss of visual acuity. Use standard vitreoretinal surgical techniques during ENCELTO implantation to minimize the risk of retinal tears and retinal detachment. Monitor for any signs or symptoms of retinal tear and/or retinal detachment. Treat rhegmatogenous retinal detachment and retinal tears promptly. Remove ENCELTO implant, if vitrectomy with a complete gas fill or silicone oil fill is required [see Dosage and Administration (2.3)].
5.4 Vitreous Hemorrhage
Vitreous hemorrhage, which may result in temporary vision loss, has occurred following ENCELTO implantation [see Adverse Reactions (6)]. Patients receiving antithrombotic medication (e.g., oral anticoagulants, aspirin, nonsteroidal anti-inflammatory drugs) may be at increased risk of vitreous hemorrhage. To reduce the risk of vitreous hemorrhage, interrupt antithrombotic medications prior to the ENCELTO implantation. Vitrectomy surgery may be necessary to clear severe, recurrent, or non-clearing vitreous hemorrhage. If the patient has a late onset vitreous hemorrhage (greater than one year following ENCELTO implantation surgery), examine the ENCELTO implantation site for possible implant extrusion. If implant extrusion has occurred, surgically reposition ENCELTO [see Implant Extrusion (5.5)].
5.5 Implant Extrusion
Implant extrusion through the initial scleral wound has occurred following ENCELTO implantation [see Adverse Reactions (6)]. Signs and symptoms of implant extrusion include recurrent uveitis, vitreous hemorrhage, eye pain more than one year after implantation, or visibility of titanium fixation loop under the conjunctiva. To reduce the risk of implant extrusion, carefully follow the specific surgical steps for ENCELTO implantation [see Dosage and Administration (2.2)].
Evaluate patients after 6 months to confirm proper positioning of ENCELTO and then annually. If ENCELTO begins to extrude, surgically reposition ENCELTO to a proper scleral wound depth either in the same site or in the opposing inferior quadrant of the vitreous cavity.
5.6 Cataract Formation
Cataract formation, including cataract cortical, cataract nuclear, cataract subcapsular, cataract traumatic, and lenticular opacities, has occurred following ENCELTO implantation [see Adverse Reactions (6)]. To reduce the risk of ENCELTO-related cataract formation or progression, carefully follow the specific surgical steps for ENCELTO implantation [see Dosage and Administration (2.2)].
5.7 Suture Related Complications
Suture related complications, including conjunctival erosions due to suture tips and suture knots, have occurred following ENCELTO implantation [see Adverse Reactions (6)]. To mitigate the risk of suture related complications, carefully follow the specific surgical steps for ENCELTO implantation [see Dosage and Administration (2.2)] and manage suture-related complications as clinically indicated.
5.8 Delayed Dark Adaptation
Delayed Dark Adaptation, a delay in the ability to adjust vision from a bright lighting condition to a dim lighting, has occurred following ENCELTO administration which remained unchanged for the duration of study follow up [see Adverse Reactions (6)]. Advise patients to take caution while driving and navigating in the dark.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflects exposure to ENCELTO in two clinical trials, Study 1 (NTMT-03-A) and Study 2 (NTMT-03-B) and are pooled for analysis. A total of 117 patients received ENCELTO, and 111 patients underwent a sham procedure and were followed for a duration of 24 months [see Clinical Studies (14)].
Serious adverse reactions occurred in six patients (5%) including suture related complications (n=5) and implant extrusion (n=1).
Table 1 lists the most common adverse reactions that occurred in >2% patients and with higher frequency in ENCELTO group compared to Sham group in Study 1 and Study 2.
|
Adverse Reactions |
ENCELTO (N=117) n (%) |
Sham (N=111) n (%) |
|
Conjunctival hemorrhage |
36 (31) |
29 (26) |
|
Delayed dark adaptation |
27 (23.1) |
1 (1) |
|
Foreign body sensation in eyes |
18 (15) |
15 (13.5) |
|
Eye pain |
18 (15) |
10 (9) |
|
Suture related complication** |
18 (15.4) |
3 (2.7) |
|
Miosis |
18 (15.4) |
0 (0.0) |
|
Conjunctival hyperemia |
13 (11) |
9 (8) |
|
Eye pruritus |
10 (9) |
4 (3.6) |
|
Ocular discomfort |
10 (9) |
1 (1) |
|
Vitreous hemorrhage |
10 (8.5) |
0 (0.0) |
|
Vision blurred |
8 (7) |
4 (4) |
|
Headache |
8 (7) |
1 (1) |
|
Dry eye |
7 (6) |
2 (2) |
|
Eye irritation |
6 (5.1) |
2 (2) |
|
Cumulative cataract incidence |
6 (5) |
0 (0) |
|
Vitreous floaters |
6 (5) |
0 (0.0) |
|
Severe visual loss>15 letters*** |
4 (3) |
0 (0) |
|
Eye discharge |
4 (3.4) |
1 (0.9) |
|
Anterior chamber cell |
4 (3.4) |
0 (0.0) |
|
Iridocyclitis |
3 (2.6) |
0 (0) |
* Pooled data from Study 1 and Study 2; Adverse reaction rates were comparable between the two studies
**Suture related complications include exposed suture, foreign body sensation, conjunctival wound dehiscence, painful sutures, suture irritation, suture granuloma, scleral wound opening, and itchy suture
*** Includes one case of visual loss due to cataract formation which remained unresolved at the end of the study
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no data on the use of ENCELTO in pregnant women. Endogenous CNTF is naturally found in maternal plasma, placental cells, and umbilical cord blood. It is not known if the use of ENCELTO increases CNTF above naturally occurring levels in these tissues.
In animal reproduction studies, subcutaneous administration of rhCNTF to pregnant rats and rabbits demonstrated no evidence of teratogenic effects on the fetus. However, when administered to rabbits at a dose level of 10ug/kg/day, a decrease in implantations and live fetuses was observed. When administered to rats at a dose level of 100ug/kg/day a decrease in corpora lutea was observed.
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
See Risk Summary for details on data.
8.2 Lactation
Risk Summary
There is no data on the presence of ENCELTO in human milk, its effects on the breastfed infant, or its impact on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ENCELTO and any potential adverse effects on the breastfed infant from rhCNTF or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ENCELTO have not been established in pediatric patients.
8.5 Geriatric Use
There were 38 patients (32%) 65 years of age and older and two patients (1%) 75 years of age and older in Study 1 and Study 2 who received ENCELTO [see Clinical Studies (14)]. Clinical studies of ENCELTO did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients.
11. Encelto Description
ENCELTO (revakinagene taroretcel-lwey) implant, is single-dose, sterile, nonpyrogenic and retrievable.
ENCELTO is an allogeneic encapsulated cell-based gene therapy that contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing recombinant human ciliary neurotrophic factor (rhCNTF) (NTC-201-6A cell line) for surgical intravitreal placement.
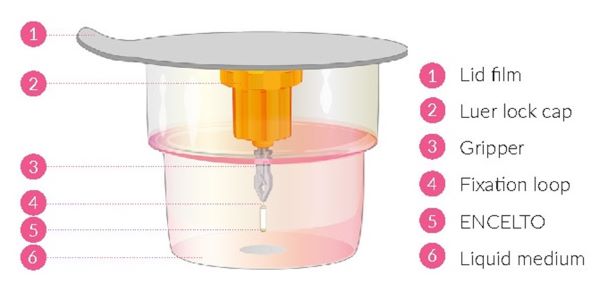
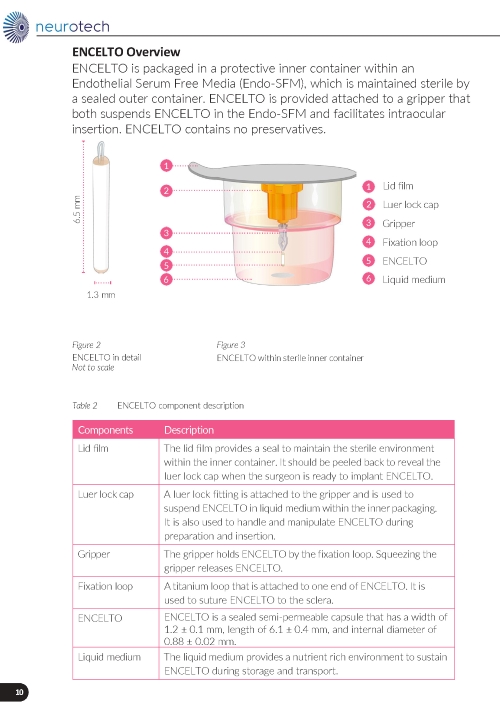
ENCELTO consists of an opaque, semi-permeable white to off-white capsule surrounding a scaffold of polyethylene terephthalate (PET) yarn, loaded with rhCNTF secreting allogeneic retinal pigment epithelial cells (NTC-201-6A cell line). Each end of the semi-permeable capsule is sealed with medical grade methacrylate adhesive, and to one end a titanium fixation loop is attached. ENCELTO width is 1.2 ± 0.1 mm, length is 6.1 ± 0.4 mm, and its internal diameter is 0.88 ± 0.02 mm (Figure 17).
ENCELTO is packaged in a protective inner container within an orange to pink liquid hold medium referred to as Endothelial Serum Free Media (Endo-SFM), which is maintained sterile by a sealed outer container. ENCELTO is provided attached, by the fixation loop, to a gripper that both suspends ENCELTO in the Endo-SFM and facilitates intraocular insertion. The Endo-SFM within the packaging inner container may contain visible particles generally described as fiber, solid, white, or metallic in appearance.
ENCELTO is manufactured using animal and human derived reagents.
12. Encelto - Clinical Pharmacology
12.1 Mechanism of Action
ENCELTO secretes recombinant human ciliary neurotrophic factor (rhCNTF), which is one of several neurotrophic factors endogenously produced by neurons and supporting glial cells. Exogenous CNTF is thought to initially target Müller glia to trigger a cascade of signaling events that may promote photoreceptor survival; however, the mechanism of action for ENCELTO is not completely understood.
12.3 Pharmacokinetics
Systemic exposure of rhCNTF was measured in 2 distribution studies in rabbits and in 2 toxicology studies in minipigs. Overall, there was no evidence of systemic exposure to rhCNTF after implantation of ENCELTO in rabbits for periods up to 9 months or in minipigs for periods of up to 6 months.
Following intraocular implantation of a single ENCELTO dose in rabbits at 12 weeks, the mean Cmax of rhCNTF in the vitreous and aqueous was 2.0 and 0.3 ng/mL, respectively, and below the level of quantitation (LLOQ) in the serum and contralateral, untreated eye. Similarly in human patients, rhCNTF levels were below the limit for LLOQ in the serum.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of ENCELTO or of other products.
In a six-month Study NTMT-02B in which patients received ENCELTO in a single eye, one out of 31 patients (3%) tested positive for serum antibodies against the ENCELTO secreted product protein rhCNTF and one patient (3%) tested positive to serum non-secreted intracellular protein DHFR.
Because of the low occurrence of anti-drug antibodies, the effect of serum anti-rhCNTF and anti-DHFR antibodies on the safety or efficacy of ENCELTO is unknown.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
No carcinogenicity or mutagenicity studies have been conducted with rhCNTF.
Impairment of Fertility
In male rats, fertility was unaffected at subcutaneous doses of rhCNTF up to 300 μg/kg/day.
See Pregnancy (8.1) for data regarding effects on female fertility.
14. Clinical Studies
The efficacy of ENCELTO was evaluated in two studies, Study NTMT-03-A (NCT03316300; Study 1) and Study NTMT-03-B (NCT03319849; Study 2) as described below.
Study 1
Study 1 was a randomized, multi-center, sham-controlled study which enrolled adults with MacTel. For enrollment, the patients were required to have a photoreceptor inner segment/outer segment (IS/OS PR) break (loss) in ellipsoid zone (EZ) between 0.16 and 2.00 mm2 measured by spectral domain-optical coherence tomography (SD-OCT) and best corrected visual acuity (BCVA) of 54-letter score or better (20/80 or better) as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at screening. Patients with neovascular MacTel were excluded. Patients were randomized to receive either ENCELTO intravitreal implant or sham procedure under standard operative procedures. Patients in ENCELTO group underwent conjunctival peritomy, implant placement in the vitreous cavity via sclerotomy and closure with sutures. Patients in the Sham group underwent conjunctival peritomy, scleral pressure, and conjunctival closure with sutures. One hundred and fifteen (96%) of 120 patients underwent the assigned procedure and were included in the analysis of efficacy.
A total of 120 patients were randomized and of these, 115 patients (ENCELTO group: 58; Sham group: 57) comprise the efficacy analysis population. The demographic characteristics of the efficacy analysis population were as follows: the mean age was 61 years (range 40 to 78 years), 79 patients (69%) were female, 98 patients (85%) were White, 5 patients (4%) were Asian, 3 patients (3%) were Black or African American, 1 patient (1%) was American Indian, and 8 patients (7%) were of “other” race. Six patients (5%) were Hispanic. The median (min, max) baseline EZ area loss was 0.35 (0.15, 1.99) mm2 for the ENCELTO group and 0.36 (0.16, 1.7) mm2 for the Sham group. The median (min, max) baseline aggregate sensitivity of microperimetry within the EZ break area 35.2 (0.75, 398.8) dB for the ENCELTO group and 35.5 (2, 281.3) dB for the Sham group.
The primary efficacy outcome measure was the rate of change in the area of EZ loss (IS/OS, macular PR loss) over 24 months, as measured by SD-OCT. The secondary outcome measure was the mean change in aggregate sensitivity loss of microperimetry within the EZ break area from baseline to Month 24.
The efficacy outcome results for Study 1 are summarized in Table 2.
|
Efficacy endpoints |
ENCELTO n= 58 |
Sham n=57 |
Difference ENCELTO-Sham |
P-valuec |
|
Rate of change in EZ area loss from baseline over 24 monthsa mm2 (95% CI) |
0.075 (0.05, 0.10) |
0.166 (0.14, 0.19) |
-0.091 (-0.13, -0.06) |
<0.0001 |
|
Mean change in aggregate retinal sensitivity loss from baseline to 24-monthsb dB (95% CI) |
25.27 (15.88, 34.67) |
43.02 (31.78, 54.26) |
-17.75 (-32.58, -2.91) |
0.02 |
CI = confidence interval, EZ=ellipsoid zone
a Estimated by using a longitudinal mixed model including EZ area loss as the dependent variable, patient-specific random intercepts, treatment group, time (continuous), and interaction between treatment and time as covariates. The baseline and Months 12, 16, 20, and 24 visits were included.
b Estimated by using two-sample t-test; seven ENCELTO and four Sham patients were excluded due to missing data.
c Statistically significant at two-sided alpha of 0.05.
Study 2
Study 2 was a randomized, multi-center, sham-controlled study which enrolled adult with MacTel. For enrollment, the patients were required to have an IS/OS PR break in EZ between 0.16 and 2.00 mm2 measured by SD-OCT and BCVA of 54-letter score or better (20/80 or better) as measured by the ETDRS chart at screening. Patients with neovascular MacTel were excluded.
Patients were randomized to receive either ENCELTO intravitreal implant or sham procedure under standard peri-operative procedures. Patients in ENCELTO group underwent conjunctival peritomy, implant placement in the vitreous cavity via sclerotomy and closure with sutures. Patients in the Sham group underwent conjunctival peritomy, scleral pressure, and conjunctival closure with sutures. One hundred and thirteen (95%) of the 119 patients underwent the assigned procedure and were included in efficacy evaluation.
A total of 119 patients were randomized and of these, 113 patients (ENCELTO group: 59; Sham group: 54) comprise the efficacy analysis population. The demographic characteristics of the efficacy analysis population were as follows: the mean age was 59 years (range: 40 to 75 years), 82 patients (73%) were female, 102 patients (90%) were White, 4 patients (4%) were Asian, and 7 patients (6%) were of “other” race or “unable to specify” race. Eight patients (7%) were Hispanic. The median (min, max) baseline EZ area loss was 0.48 (0.16, 1.63) mm2 for the ENCELTO and 0.39 (0.16, 1.38) mm2 for the Sham group. The median (min, max) baseline aggregate sensitivity of microperimetry within the EZ break area 40.07 (4.82, 291.52) dB for the ENCELTO group and 28.86 (0.33, 221.17) dB for the Sham group.
The primary efficacy outcome measure was the rate of change in the area of EZ loss (IS/OS, macular PR loss) over 24 months, as measured by SD-OCT. The secondary outcome measure was the mean change in aggregate sensitivity loss of microperimetry within the EZ break area from baseline to Month 24.
The efficacy results from Study 2 are summarized in Table 3 below.
|
Efficacy endpoints |
ENCELTO n= 59 |
Sham n=54 |
Difference ENCELTO-Sham |
P-valuec |
|
Rate of change in EZ area loss from baseline over 24 monthsa mm2 (95% CI) |
0.111 (0.08, 0.14) |
0.160 (0.13, 0.19) |
-0.049 (-0.089, -0.008) |
0.0186c |
|
Mean change in aggregate retinal sensitivity loss from baseline to 24-monthb dB (95% CI) |
40.02 (26.08, 53.96) |
41.97 (30.34, 53.60) |
-1.95 (-20.33, 16.43) |
0.83 |
CI = confidence interval, EZ=ellipsoid zone
a Estimated by using a longitudinal mixed model including EZ area loss as the dependent variable, patient-specific random intercepts, treatment group, time (continuous), and interaction between treatment and time as covariates. The baseline and Months 12, 16, 20, and 24 visits were included.
b Estimated by using two-sample t-test; Seven ENCELTO and six Sham patients were excluded due to missing data.
c Statistically significant at two-sided alpha of 0.05.
16. How is Encelto supplied
16.1 How Supplied
ENCELTO is supplied as a sterile, single-dose, implant that contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing rhCNTF (NTC-201-6A cell line).
ENCELTO is packaged in a protective inner container within an Endothelial Serum Free Media (Endo-SFM), which is maintained sterile by a sealed outer container. ENCELTO is provided attached to a gripper that both suspends ENCELTO in the Endo-SFM and facilitates intraocular insertion. ENCELTO contains no preservatives.
NDC: 82958-501-01
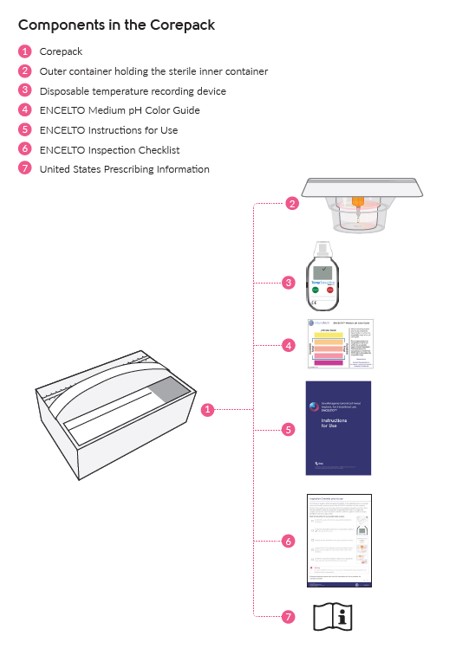
See Table 4, (Figure 16) and ENCELTO “Instructions for Use” for additional details.
|
Components |
Description |
|
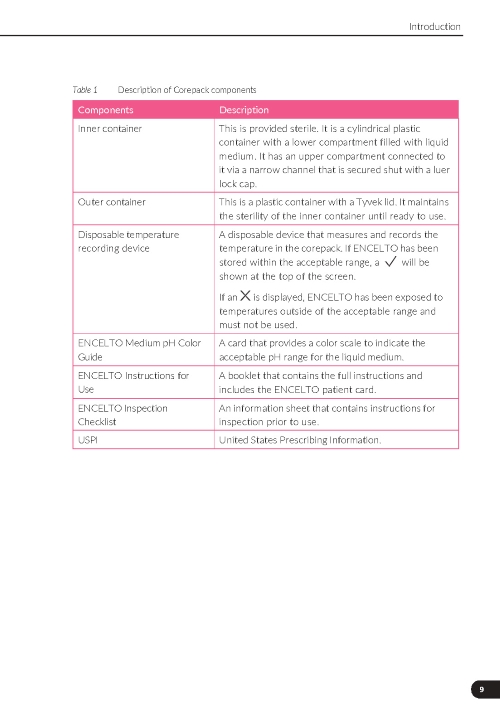
Inner container |
This is provided sterile. It is a cylindrical plastic container with a lower compartment filled with liquid medium. It has an upper compartment connected to it via a narrow channel that is secured shut with a luer lock cap. |
|
Outer container |
This is a plastic container with a foil lid hermetically sealed. It maintains the sterility of the inner container until ready to use. |
|
Disposable temperature recording device |
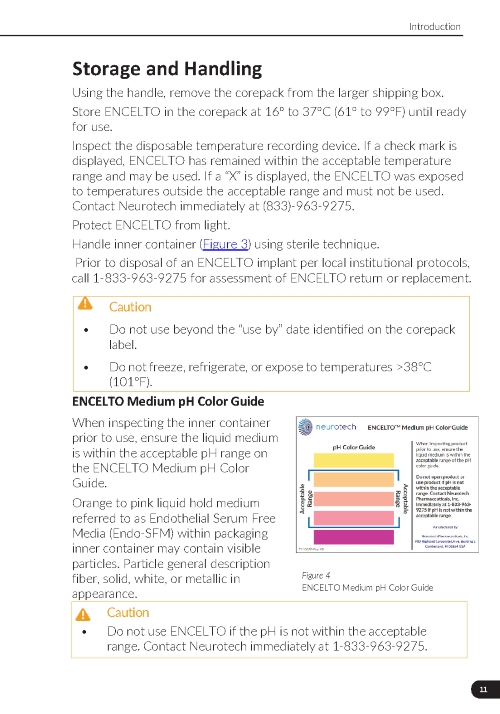
A disposable device that measures and records the temperature in the package. If ENCELTO has been stored within the acceptable range, a “✓” will be shown at the top of the screen. If an “X” is displayed, ENCELTO has been exposed to temperatures outside of the acceptable range and must not be used. |
|
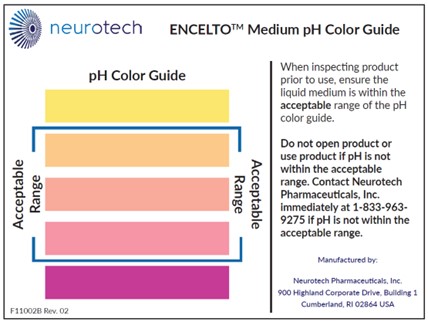
ENCELTO Medium pH Color Guide |
A card that provides a color scale to indicate the acceptable pH range for the liquid medium. |
|
ENCELTO Instructions for Use |
A booklet that contains the full instructions and includes the ENCELTO patient card. |
|
ENCELTO Inspection Checklist |
An information sheet that contains instructions for inspection prior to use. |
|
USPI |
United States Prescribing Information. |
Figure 16. ENCELTO Corepack Contents
Figure 17. ENCELTO
Figure 18. ENCELTO Inner Container
Figure 19. pH Color Guide
16.2 Storage and Handling
1. Using the handle, remove the corepack from the larger shipping box (Figure 16).
2. Store ENCELTO in the corepack at 16º to 37ºC (61º to 99ºF) until ready for use.
3. Do not freeze or refrigerate.
4. Inspect the disposable temperature recording device. If a check mark is displayed, the ENCELTO has remained within the acceptable temperature range and may be used. If a “X” is displayed, the ENCELTO was exposed to temperatures outside the acceptable range and must not be used. Contact Neurotech immediately at (833)-963-9275.
• Protect ENCELTO from light.
5. Handle inner container (Figure 18) using sterile technique.
6. Do not use beyond the “use by” date identified on the corepack label.
7. Do not use ENCELTO if the pH is not within the acceptable range (Figure 19). Contact Neurotech immediately at (833)-963-9275.
• Prior to disposal of an ENCELTO implant per local institutional protocols, call 1-833-963-9275 for assessment of ENCELTO return or replacement.
Orange to pink liquid hold medium referred to as Endothelial Serum Free Media (Endo-SFM) within packaging inner container may contain visible particles. Particle general description fiber, solid, white, or metallic in appearance.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Discuss the following with the patient.
Advise patients that ENCELTO implantation may be associated with infectious endophthalmitis (eye infection), retinal tear and detachment (retina separates from the eye wall resulting in vision loss), vitreous hemorrhage (bleeding within the central cavity of the eye), implant extrusion, suture-related complications, cataract formation (clouding of the lens of the eye), severe vision loss, and delayed dark adaptation (ability of the eye to adjust from bright lighting conditions to dark lighting conditions) [see Warnings and Precautions (5)].
Instruct patients to seek immediate care from an ophthalmologist if they experience any signs or symptoms that could be associated with these events which may include the following:
- An increase in floaters, the appearance of “spider webs”, flashing lights, sensitivity to light, or loss of vision or visual field;
- Increasing eye pain, progressive redness in the white of the eye, a sudden sensation that something is in their eye (i.e., foreign body sensation) or eye discharge.
Advise patients that they may temporarily experience the following after ENCELTO implantation:
- Mild sensation of something in the eye (i.e., foreign body sensation)
- Eye redness, irritation, pain or discomfort, or dryness
- Blurred vision or floaters
Advise patients that delayed dark adaptation may be experienced for the length of time that ENCELTO is surgically placed [see Warnings and Precautions (5.8)]. Advise patients on the following safety precautions.
- Driving: delayed dark adaptation may impair one's ability to see objects, pedestrians, or road signs when moving rapidly from a brightly lit environment to a dimly lit environment (for example, entering a tunnel during the daytime).
- Navigating in the dark: Advise caution when moving from bright to dark areas, such as entering a dark room or stepping outside at dusk. Consider using flashlights, nightlights, or motion-activated lighting at home.
- Consider wearing sunglasses or tinted lenses in bright environments to reduce the impact of transitioning from light to dark.
Magnetic Resonance (MR) Conditional Information

ENCELTO is MR conditional. Advise patients that they have ENCELTO implanted in their eye and provide the patient with their implant card should they require Magnetic Resonance Imaging (MRI).
Driving and Using Machines
- Advise patients to not drive or use machinery until the eye shield has been removed and their ophthalmologist informs them that their vision has recovered to an acceptable level.
Postoperative Care
Advise patients on the following post operative care:
- Avoid heavy lifting (over 20 pounds) for one week.
- Keep water out of the eye (e.g., close eye while showering) for one week.
- Protect eyes by wearing glasses or protective eyewear during the day and using an eye shield at night for one week.
- Use a topical antibiotic solution at a frequency of 1 drop four times a day for 7 days.
- Use a steroid drop taper of prednisolone acetate 1% (or equivalent) starting the day after surgery with the following taper:
- 1 drop four times a day for the first 7 days;
- 1 drop three times a day for the next 7 days;
- 1 drop two times a day for the next 7 days;
- 1 drop once a day for the last 7 days.
Manufactured by:
Neurotech Pharmaceuticals, Inc.
Building 1, Suite 101
Cumberland, RI 02864
U.S. license number: 2321
|
PATIENT INFORMATION ENCELTO (En-SEL-toh) A surgical implant for use in the eye |
|
What is ENCELTO? ENCELTO is an encapsulated cell-based gene therapy. It is a small capsule, about the size of a grain of rice, that is placed inside the eye to release a protein called recombinant human ciliary neurotrophic factor (rhCNTF) that can directly reach the retina, the light sensitive part of the eye. The capsule contains living cells that have been genetically modified to continuously produce and release CNTF. This protein helps protect certain cells in your retina, supporting their health and reducing the loss of light-sensing cells known as photoreceptors. ENCELTO is used to treat adults with idiopathic macular telangiectasia type 2 (MacTel); a retinal disease that causes progressive vision loss. Your ophthalmologist will assess your vision and review your medical history to determine if ENCELTO is the right treatment for you. |
|
Who should not receive an ENCELTO surgical implant? ENCELTO has not been tested in pediatric patients or pregnant women. The outpatient surgical procedure should not be performed if you are currently experiencing an active or suspected eye infection. You should not receive ENCELTO if you have a known hypersensitivity to Endothelial Serum Free Media (Endo-SFM) |
|
Before receiving ENCELTO, tell your ophthalmologist about all your medical conditions, including:
|
|
How is ENCELTO administered? ENCELTO is inserted into the eye as an outpatient surgical procedure performed by an ophthalmologist experienced in retinal surgery. If removal of ENCELTO is necessary, the removal surgery must also be done by an ophthalmologist experienced in vitreoretinal surgery in an operating room as an outpatient surgery. |
|
What should I avoid after placement of ENCELTO? Immediately post-operative:
Post operative care:
Magnetic Resonance (MR) Conditional Information
IMPORTANT: ENCELTO is MR conditional. You will receive an implant card, which should be shown to your imaging technician if you need Magnetic Resonance Imaging (MRI) at any time while the ENCELTO implant is in your eye. The card will include details about the ENCELTO implant, the date of insertion, and on the back, instructions for the imaging technician to access important MRI safety information. Call your ophthalmologist for medical advice about side effects. You may report side effects to the Food and Drug Administration (FDA) at 1-800-FDA-1088. |
|
What are the possible side effects of ENCELTO? Please follow all post-operative instructions given by your ophthalmologist and ensure you attend all follow-up visits as recommended. Potential side effects Please be advised that ENCELTO and the surgical insertion has related risks such as, but not limited to, endophthalmitis (eye infection), retinal tear and detachment (retina tears and potentially separates from the eye wall resulting in vision loss), vitreous hemorrhage (bleeding within the central cavity of the eye), implant extrusion (the ENCELTO begins to work it’s way out of the eye), suture related issues (such as suture related eye irritation or exposure of sutures), temporary or permanent loss of vision, accelerated cataract formation (clouding of the lens of the eye), and delayed dark adaptation (the ability of the eye to adjust from bright lighting conditions to dark lighting conditions). If delayed dark adaptation occurs, it is unknown for how long these symptoms will be experienced. Take the following safety precautions.
It is common to experience the following symptoms following ENCELTO surgery:
When to Seek Ophthalmologist Advice You are to seek immediate care from an ophthalmologist if there are sudden changes in your vision, such as an increase in floaters, the appearance of “spider webs,” flashing lights, sensitivity to light, loss of vision or visual field, progressively worsening eye pain, or increasing discharge/drainage from the eye as these symptoms could be a sign of a more serious issue. |
|
General information about the safe and effective use of ENCELTO Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your ophthalmologist for a copy of the information about ENCELTO that is written for healthcare professionals. You should inform your ophthalmologist that you have an ENCELTO implant inserted prior to any eye examination. |
|
What are the ingredients in ENCELTO? Active ingredients: ENCELTO is an encapsulated cell-based therapy that contains 200,000 to 440,000 allogeneic retinal pigment epithelial cells expressing rhCNTF (NTC-201-6A cell line), a neurotrophic factor. Inactive ingredients: ENCELTO contains excipients (Human Endothelial Serum Free Medium (SFM) that may cause sensitivity in some patients.
Neurotech Pharmaceuticals, Inc. Building 1, Suite 101 Cumberland, Rhode Island, 02864 |
This Patient Information has been approved by the U.S. Food and Drug Administration. F11002F Rev. 01
Revised: 2025/03
| ENCELTO
revakinagene taroretcel-lwey implant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Neurotech Pharmaceuticals, Inc. (117685116) |