Coagadex: Package Insert / Prescribing Info
Package insert / product label
Generic name: coagulation factor x human
Dosage form: kit
Drug class: Miscellaneous coagulation modifiers
J Code (medical billing code): J7175 (Per unit, injection)
Medically reviewed by Drugs.com. Last updated on Nov 4, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
COAGADEX® (Coagulation Factor X (Human))
Lyophilized Powder for Solution for Intravenous Injection
Initial U.S. Approval: [2015]
Indications and Usage for Coagadex
COAGADEX, Coagulation Factor X (Human), is a plasma-derived human blood coagulation factor indicated in adults and children with hereditary Factor X deficiency for:
Coagadex Dosage and Administration
For intravenous use only after reconstitution.
- Each vial of COAGADEX contains the labeled amount of Factor X in international units (IU) (2)
- The dosage and duration of treatment depend on the severity of the Factor X deficiency, the location and extent of the bleeding and the patient's clinical condition (2.1)
- For prophylaxis of bleeding episodes (2.1):
| Age | Initial dose | Further management |
|---|---|---|
| Children: Less than 12 years of age | 40 IU/kg twice weekly | Monitor trough blood levels of Factor X targeting ≥5 IU/dL and adjust dosage to clinical response and trough levels. Do not exceed a peak level of 120 IU/dL. |
| Adults and adolescents: 12 years of age or older | 25 IU/kg twice weekly |
- For treatment of bleeding episodes (2.1):
| Age | Initial dose | Further management |
|---|---|---|
| Children: Less than 12 years of age | 30 IU/kg | Infuse at first sign of bleeding. Repeat at intervals of 24 hours until the bleed stops. |
| Adults and adolescents: 12 years of age or older | 25 IU/kg |
- For perioperative management (2.1):
| Age | Initial dose | Further management |
|---|---|---|
| Children: Less than 12 years of age | Use a factor of 0.6 to calculate the required dose | Pre-surgery: raise plasma Factor X levels to 70-90 IU/dL. Post-surgery: maintain plasma Factor X levels at ≥50 IU/dL until the patient is no longer at risk of bleeding due to surgery. |
| Dose (IU) = body weight (kg) × desired factor X rise† (IU/dL or % of normal) × 0.6 | ||
| Adults and adolescents: 12 years of age or older | Use a factor of 0.5 to calculate the required dose | |
| Dose (IU) = body weight (kg) × desired factor X rise‡ (IU/dL or % of normal) × 0.5 | ||
Dosage Forms and Strengths
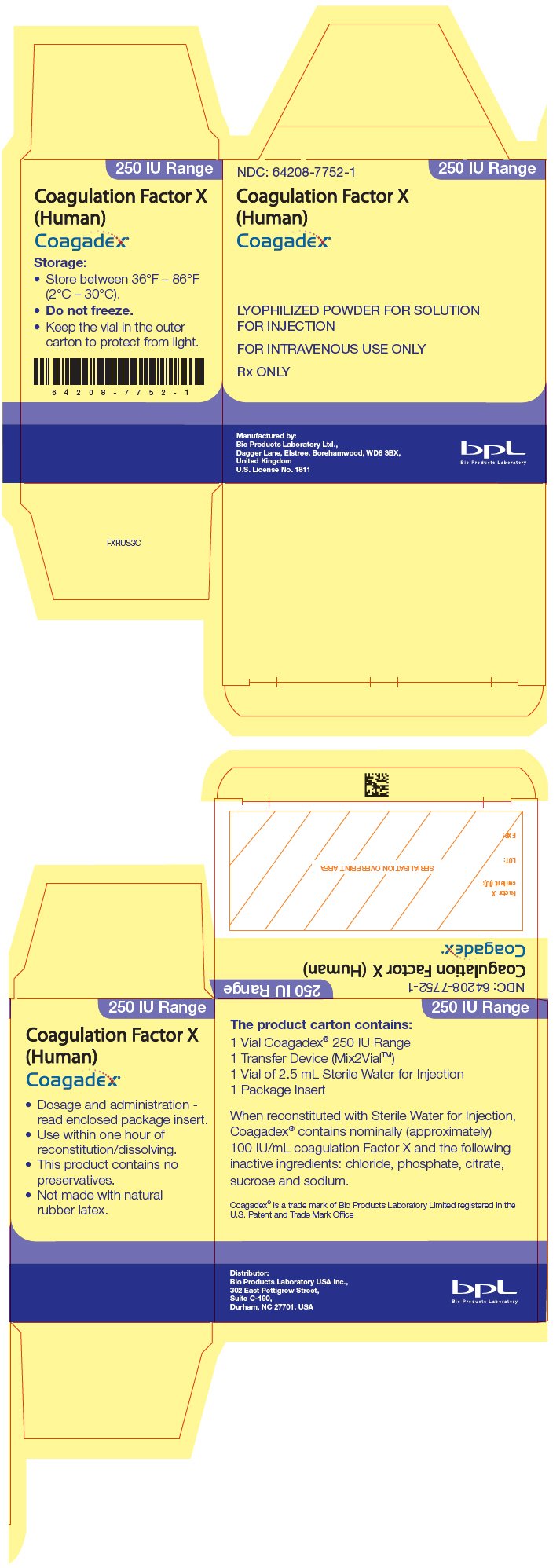
COAGADEX is available as a lyophilized powder for reconstitution in single-dose vials containing nominally (approximately) 250 IU or 500 IU of Factor X activity. When reconstituted using the Sterile Water for Injection supplied with the kit, the final concentration is approximately 100 IU/mL (3).
Contraindications
Do not use in patients who have had life-threatening hypersensitivity reactions to COAGADEX (4).
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, are possible. Should symptoms occur, discontinue COAGADEX and administer appropriate treatment (5.1).
- Development of neutralizing antibodies (inhibitors) may occur. If expected plasma Factor X activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures Factor X inhibitor concentration (5.2).
- COAGADEX is made from human blood and therefore carries a risk of transmitting infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent (5.4).
Adverse Reactions/Side Effects
The most common adverse drug reactions (frequency ≥ 5% of subjects) observed in clinical trials were infusion site erythema, infusion site pain, fatigue and back pain (6).
To report SUSPECTED ADVERSE REACTIONS, contact Kedrion Biopharma, Inc. at 1-855-3KDRION (1-855-353-7466) or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2024
Full Prescribing Information
1. Indications and Usage for Coagadex
COAGADEX, Coagulation Factor X (Human), is a plasma-derived human blood coagulation Factor indicated in adults and children with hereditary Factor X deficiency for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding in patients with mild, moderate, and severe hereditary Factor X deficiency
2. Coagadex Dosage and Administration
For intravenous use after reconstitution only
2.1 Dose
- Dose and duration of the treatment depend on the severity of the Factor X deficiency, location and extent of the bleeding, the patient's age (<12 years or >12 years) and the patient's clinical condition.
- Base the dose and frequency on the individual clinical response. Do not administer more than 60 IU/kg daily.
- Each vial of COAGADEX is labeled with the actual Factor X potency/content in International Units (IU).
| Age | Initial dose | Further management |
|---|---|---|
| Children: Less than 12 years of age | 40 IU/kg twice weekly | Due to inter-and intra-patient variability, it is recommended that trough blood levels of Factor X should be monitored at intervals, especially in the first weeks of therapy or after dosages changes. Adjust dosage regimen to clinical response and trough levels of Factor X of at least 5 IU/dL. Do not exceed a peak level of 120 IU/dL. [For more detailed calculations of dose, see Detailed Dose Calculation (2.1)]. |
| Adults and adolescents: 12 years of age or older | 25 IU/kg twice weekly |
| Age | Initial dose | Further management |
|---|---|---|
| Children: Less than 12 years of age | 30 IU/kg | Infuse COAGADEX when the first sign of bleeding occurs [see Clinical Trial Experience (6.1)]. Repeat at intervals of 24 hours until the bleed stops. [For more detailed calculations of dose, see Detailed Dose Calculation (2.1)]. |
| Adults and adolescents: 12 years of age or older | 25 IU/kg |
| Age | Initial dose | Further management |
|---|---|---|
| Children: Less than 12 years of age | Use a factor of 0.6 to calculate the required dose | Measure post-infusion plasma Factor X levels for each patient before and after surgery to ensure that hemostatic levels are obtained and maintained. Pre-surgery: calculate the dose of COAGADEX to raise plasma Factor X levels to 70-90 IU/dL Post-surgery: Repeat dose as necessary to maintain plasma Factor X levels at a minimum of 50 IU/dL until the patient is no longer at risk of bleeding due to surgery |
| Dose (IU) = body weight (kg) × desired factor X rise† (IU/dL or % of normal) × 0.6 | ||
| Adults and adolescents: 12 years of age or older | Use a factor of 0.5 to calculate the required dose | |
| Dose (IU) = body weight (kg) × desired factor X rise‡ (IU/dL or % of normal) × 0.5 | ||
Detailed Dose Calculation
-
For young children (less than 12 years of age), the incremental recovery is approximately 1.7 IU/dL per IU/kg so the number at the end of each of the above formulae changes as follows:
Dose (IU) = Body Weight (kg) × Desired Factor X Rise (IU/dL) × 0.6
†The desired Factor X rise is the difference between the patient's plasma Factor X level and the maximum desired level.
To estimate the expected in vivo maximum increase in plasma Factor X, expressed as IU/dL (or % of normal), use the following formula:
Estimated Increment of Factor X (IU/dL or % of normal) = [Total Dose (IU)/Body Weight (kg)] × 1.7 -
For adolescents and adults (at least 12 years of age), the incremental recovery is approximately 2.0 IU/dL per IU/kg so the number at the end of each of the above formulae changes as follows:
Dose (IU) = Body Weight (kg) × Desired Factor X Rise (IU/dL or % of normal) × 0.5
‡The desired Factor X rise is the difference between the patient's plasma Factor X level and the maximum desired level.
To estimate the expected in vivo maximum increase in plasma Factor X expressed as IU/dL (or % of normal) use the following formula:
Estimated Increment of Factor X (IU/dL or % of normal) = [Total Dose (IU)/Body Weight (kg)]× 2
2.2 Preparation and Reconstitution
- Always work on a clean surface and wash your hands before performing the following procedures.
- To reconstitute, use the diluent (Sterile Water for Injection) and transfer device (Mix2Vial) provided in the COAGADEX carton.
- To administer, you will also need a syringe and suitable needle (not provided in the COAGADEX carton).
- Bring the vials of COAGADEX and the Sterile Water for Injection to room temperature before mixing.
- The reconstitution is performed as follows:
|
| Step 1
|
|
| Step 3
|
|
| Step 4
|
|
| Step 5
|
|
| Step 6
|
|
| Step 8
|
2.3 Administration
For intravenous administration only
- If the dose requires more than one vial of COAGADEX:
- Reconstitute each vial (steps 1 to 5) using a new Mix2Vial for each vial
- Draw up all of the solution into a single syringe (steps 6 to 8)
- Visually inspect the final solution for particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed
- Attach a suitable needle to the syringe
- Administer by intravenous infusion at a rate of 10 mL/min, but no more than 20 mL/min
3. Dosage Forms and Strengths
COAGADEX is available as a white or off-white lyophilized powder for reconstitution in single-dose vials containing nominally (approximately) 250 IU or 500 IU of Factor X activity. The exact potency/content is listed on the vial label. When reconstituted using the Sterile Water for Injection supplied with the kit, the final concentration is approximately 100 IU/mL.
Factor X activity in COAGADEX is defined in IU and determined using an in vitro chromogenic assay and a Factor X concentrate reference standard calibrated against the current World Health Organization (WHO) International Standard for Blood Coagulation Factors II and X, Concentrate.
4. Contraindications
COAGADEX is contraindicated in patients who have had life-threatening hypersensitivity reactions to COAGADEX [see Description (11)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Allergic type hypersensitivity reactions, including anaphylaxis, are possible. Early signs of hypersensitivity reactions including angioedema, infusion site inflammation (e.g. burning, stinging, erythema), chills, cough, dizziness, fever, flushing, generalized urticaria, headache, hives, hypotension, lethargy, musculoskeletal pains, nausea, pruritus, rash, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing. If hypersensitivity symptoms occur, discontinue use of the product immediately and administer appropriate emergency treatment.
COAGADEX contains traces of human proteins other than Factor X.
5.2 Neutralizing Antibodies
The formation of neutralizing antibodies (inhibitors) to Factor X may occur. Monitor all patients treated with COAGADEX for the development of inhibitors by appropriate clinical observations and laboratory tests. If expected Factor X activity levels are not attained, or if bleeding is not controlled with an expected dose, perform an assay that measures Factor X inhibitor concentration.
5.3 Transmissible Infectious Agents
As COAGADEX is made from human blood, it may carry a risk of transmitting infectious agents, e.g. viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. There is also the possibility that unknown infectious agents may be present in the product. The risk that the product will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, this product may still potentially transmit diseases.
All infections suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare providers to Kedrion Biopharma, Inc. at 1-855-3KDRION (1-855-353-7466) or US_Medicalinfo@kedrion.com
5.4 Monitoring and Laboratory Tests
- Monitor plasma Factor X activity by performing a validated test (e.g. one-stage clotting assay), to confirm that adequate Factor X levels have been achieved and maintained [see Dosage and Administration (2)].
- Monitor for the development of Factor X inhibitors. Perform a Nijmegen-Bethesda inhibitor assay if expected Factor X plasma levels are not attained, or if bleeding is not controlled with the expected dose of COAGADEX. Use Nijmegen-Bethesda Units (BU) to report inhibitor levels.
6. Adverse Reactions/Side Effects
The most common adverse drug reactions (frequency ≥ 5% of subjects) observed in clinical trials were infusion site erythema, infusion site pain, fatigue, and back pain.
6.1 Clinical Trials Experience
As clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in clinical practice.
During the clinical development of COAGADEX involving three multicenter, open-label, non-randomized clinical studies, 27 individual subjects with hereditary Factor X deficiency received at least one dose of COAGADEX.
Sixteen subjects aged 12 to 58 years with moderate to severe hereditary Factor X deficiency (basal FX:C < 5 IU/dL) received doses of COAGADEX for pharmacokinetic evaluation, on-demand treatment for control of bleeding episodes, and/or perioperative management of minor surgical or dental procedures. A total of 468 infusions were administered, including 242 for on-demand treatment and control of bleeding episodes, 6 for perioperative management and 31 for PK assessments. Spontaneous, traumatic and menorrhagic bleeding episodes were treated with an on-demand dose of 25 IU/kg for up to 2 years.
Two subjects aged 55 and 59 years with mild hereditary Factor X deficiency (basal FX:C 6 IU/dL and 8 IU/dL) received COAGADEX for perioperative management of four major surgical procedures. There were 40 exposure days to COAGADEX.
Six adverse reactions were reported in 2 of the 18 subjects. These were infusion site erythema (2 reports in 1 subject [5.6%]), fatigue (2 reports in 1 subject [5.6%]), back pain (1 report [5.6%]) and infusion site pain (1 report [5.6%]).
In a separate study, nine children (aged 2 to 11 years), of whom four were less than 6 years of age, received 537 (mean 59.7) doses of COAGADEX as routine prophylaxis of bleeding episodes during a period of at least 6 months. In addition, 22 infusions were given to treat a bleed, equivalent to 2.1 bleeds per subject per year. There were no adverse drug reactions in this study.
6.2 Immunogenicity
Immunogenicity was evaluated in three studies and all subjects (adults and children) underwent Factor X inhibitor testing (inhibitor screen and Nijmegen-Bethesda assay) at baseline, end of study and at 3-monthly intervals in between. For subjects who underwent surgery, inhibitor testing was done pre-surgery and on discharge. All inhibitor tests were negative. Additionally, comparison of pharmacokinetic (PK) parameters at the repeat PK assessment with those at first dose did not suggest development of any inhibitors to Factor X.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to COAGADEX in the studies described above with the incidence of antibodies in other studies or to other products.
7. Drug Interactions
Drug interaction studies have not been performed. Use with caution in patients who are receiving other plasma products that may contain Factor X (e.g. fresh frozen plasma, prothrombin complex concentrates). Based on the mechanism of action, COAGADEX is likely to be counteracted by direct and indirect Factor Xa inhibitors [see Clinical Pharmacology (12.1)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There is limited data with COAGADEX in pregnant women to inform on drug-associated risk. Animal reproduction studies have not been conducted using COAGADEX. It is not known whether COAGADEX can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of COAGADEX in human milk, the effects on the breast-fed infant, or the effects on milk production. The developmental and health benefits of breast-feeding should be considered along with the mother's clinical need for COAGADEX and any potential adverse effects on the breast-fed infant from COAGADEX or from the underlying maternal condition.
8.4 Pediatric Use
A prospective, open-label, multicenter study was conducted in nine pediatric patients (0-5 years, n=4; 6-11 years, n=5) with severe (n=8) or moderate (n=1) hereditary factor X deficiency, to assess safety, efficacy, and pharmacokinetics of COAGADEX used prophylactically and on-demand. Patients had baseline FX levels < 5 IU/dL, and were dosed to achieve post-treatment levels of at least 5 IU/dL. After ≥26 weeks and ≥50 exposure days, investigators rated pdFX efficacy for preventing/decreasing bleeds. At end of study, investigators rated pdFX efficacy excellent for all subjects. Ten bleeds occurred (n = 3 subjects; 6 major, 3 minor, 1 unassessed for severity). Trough FX:C levels remained >5 IU/dL for all subjects after achievement of steady state. All AEs were unrelated to treatment; no inhibitor development or clinically significant changes in laboratory parameters were observed.
Six additional pediatric subjects aged 12 to 17 years were included in a prospective, open-label, multicenter study of COAGADEX to treat spontaneous, traumatic and menorrhagic bleeding episodes. Overall efficacy and safety outcomes were comparable to those in the younger age groups. [See Clinical Trial Experience (6.1), Pharmacokinetics (12.3) and Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of COAGADEX did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10. Overdosage
One case of accidental overdosage was reported in the clinical trials, in which a subject received approximately 80 IU/kg Factor X to treat a bleed. No adverse events were reported relating to this overdose.
11. Coagadex Description
COAGADEX is a plasma-derived, sterile, purified concentrate of human coagulation Factor X that contains sucrose as a stabilizer. It is formulated as a lyophilized powder for solution for intravenous administration. When reconstituted with Sterile Water for Injection it forms a colorless, clear or slightly pearl-like solution. COAGADEX contains nominally (approximately) 100 IU/mL of coagulation Factor X and the following inactive ingredients: chloride, phosphate, citrate, sucrose and sodium. Each vial of COAGADEX is labeled with the actual Factor X activity/content expressed in IU per vial. Factor X activity in IU is determined using an in vitro chromogenic assay and a Factor X concentrate reference standard calibrated against the current WHO International Standard for Blood Coagulation Factors II and X Concentrate.
COAGADEX contains no added biological components such as heparin, albumin or anti-thrombin. The content of Factor II and Factor IX are ≤ 1 IU/mL of the reconstituted product. Factor Xa and Factor IIa were not detectable by Non-activated Partial Thromboplastin Time (NaPTT) or Fibrinogen Clotting Time (FCT) tests for potential thrombogenicity, and comprised < 10 parts per million by weight when tested by more sensitive analytical methods. The specific activity of COAGADEX is typically 80-137 IU per mg protein. The product contains no preservatives.
COAGADEX is manufactured from plasma, obtained from healthy US donors, who have passed viral screening tests. All donors are subjected to medical examinations, laboratory tests, and a review of their medical history before being allowed to donate blood or plasma.
All plasma donations are screened for antibody to human immunodeficiency virus (HIV)-1/2, hepatitis C virus (HCV), and hepatitis B surface antigen (HBsAg). Additional testing of donations is carried out in plasma mini-pools (512 donations per pool) with nucleic acid amplification testing (NAT) for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis A virus (HAV) and human parvovirus B19. Furthermore, each manufacturing pool is tested to be negative for HBsAg and anti-HIV-1/2 antibodies. Also, manufacturing pools are non-reactive by nucleic acid test for HAV, HBV, HCV, and HIV-1. The limit for human parvovirus B19 in the manufacturing pools is set not to exceed 104 IU/mL.
Three processing steps specifically designed to remove or inactivate viruses are:
1) Solvent/detergent treatment targeted to inactivate enveloped viruses
2) A 15-nm filtration step designed to remove small viruses including non-enveloped viruses
3) Terminal dry-heat treatment at 80°C for 72 hours in the final container to inactivate enveloped and non-enveloped viruses
The capacity of the manufacturing process to remove and/or inactivate enveloped and non-enveloped viruses has been validated by laboratory spiking studies on a scaled-down process model. Overall virus reduction was calculated only from steps that were mechanistically independent from each other. Table 2 presents the contribution of each process step to virus reduction and the overall process reduction.
| Process Log10 Reduction of Virus (LRV) over manufacturing step | ||||||
|---|---|---|---|---|---|---|
| Virus | Type (Envelope/Genome) | Size (nm) | Solvent/detergent | 15-nm filtration | Terminal dry-heat treatment | Total LRV |
| HIV: Human immunodeficiency virus | ||||||
| SIN: Sindbis virus, model for HCV | ||||||
| WNV: West Nile Virus | ||||||
| BVDV: Bovine viral diarrhea virus, model for HCV | ||||||
| IBR: Infectious bovine rhinotracheitis, bovine herpes virus model for enveloped DNA viruses including HBV | ||||||
| HAV: Hepatitis A virus | ||||||
| HSV: Herpes simplex virus | ||||||
| CPV: Canine parvovirus, model for human parvovirus B19 | ||||||
| NA: Not applicable, solvent/detergent treatment step is limited to the inactivation of enveloped viruses | ||||||
| NT: Not tested | ||||||
|
||||||
| HIV | Env/RNA | 80-100 | > 4.6 | > 6.8 | 5.5 | > 16.9 |
| SIN | Env/RNA | 60-70 | 6.0 | NT | NT | 6.0 |
| BVDV | Env/RNA | 50-70 | > 5.1 | > 4.5 | > 5.2 | > 14.8 |
| HSV | Env/DNA | 120-200 | > 5.4 | 5.8 | 3.5 | > 14.7 |
| IBR | Env/DNA | 120-200 | > 5.3 | NT | NT | > 5.3 |
| WNV | Env/RNA | 40-60 | 4.9 | NT | NT | 4.9 |
| HAV | Non-Env/RNA | 25-30 | NA | > 5.0 | > 6.1 | > 11.1 |
| CPV | Non-Env/RNA | 18-24 | NA | 4.3* | 4.2 | 8.5 |
12. Coagadex - Clinical Pharmacology
12.1 Mechanism of Action
COAGADEX temporarily replaces the missing Factor X needed for effective hemostasis. Factor X is an inactive zymogen, which can be activated by Factor IXa (via the intrinsic pathway) or by Factor VIIa (via the extrinsic pathway). Factor X is converted from its inactive form to the active form (Factor Xa) by the cleavage of a 52-residue peptide from the heavy chain. Factor Xa associates with Factor Va on a phospholipid surface to form the prothrombinase complex, which activates prothrombin to thrombin in the presence of calcium ions. Thrombin then acts upon soluble fibrinogen and Factor XIII to generate a cross-linked fibrin clot.
12.2 Pharmacodynamics
The administration of COAGADEX increases plasma levels of Factor X and can temporarily correct the coagulation defect in these patients, as reflected by decrease in the aPTT and PT.
12.3 Pharmacokinetics
In a clinical study of COAGADEX in subjects with severe or moderate Factor X deficiency (basal FX:C < 5 IU/dL), the pharmacokinetics of COAGADEX were assessed after intravenous infusion (mean [range] infusion rate 5.9 [1.3-17.1] mL/min) of 25 IU/kg COAGADEX. Pharmacokinetic (PK) parameters were calculated from plasma Factor X:C activity measurements after subtraction of the pre-dose value. The PK assessment was repeated at least 6 months after the first dose. The PK parameters following a single dose are summarized in Table 3. The pharmacokinetics of COAGADEX were similar following the single and repeat dosing.
| First Dose Mean (CV%) (n=16) |
|
|---|---|
| CV: Coefficient of variation | |
|
|
| Cmax (IU/mL) | 0.504 (17.2) |
| Half-life (hr) | 30.3 (22.8) |
| AUC0-144h (IU.hr/mL) | 17.1 (21.0) |
| AUC(0-∞) (IU.hr/mL) | 18.0 (20.9) |
| Vss (mL/kg) | 56.3 (24.0) |
| CL (mL/kg/hr) | 1.35 (21.7) |
| MRT (hr) | 41.8 (21.7) |
| Incremental recovery (IU/dL per IU/kg)* | 2.04 (19.5) |
Incremental recovery in children <12 years of age was assessed at baseline and 6 months after the first dose. Values are summarized in Table 4.
| Pediatric age group | Visit 1* Mean (Min-Max) | End of Study† Mean (Min-Max) |
|---|---|---|
| Aged 6-11 years (n = 5) | 1.83 (1.6–2.2) | 1.99 (1.8–2.2) |
| Aged 0-5 years (n = 4) | 1.45 (1.3–1.6) | 1.62 (1.3–1.8) |
Studies were not conducted to evaluate the impact of gender or renal/hepatic function on the pharmacokinetics of COAGADEX.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Nonclinical studies evaluating the carcinogenic or mutagenic potential of COAGADEX have not been conducted. No animal studies regarding impairment of fertility following COAGADEX dosing were conducted; however, no macroscopic or microscopic pathologies in reproductive organs were observed in rats dosed every other day with 6 times the maximum recommended clinical dose of 60 IU/kg COAGADEX for 28 days.
14. Clinical Studies
Prophylaxis of Bleeding Episodes
In a multicenter, open-label, non-randomized clinical trial, the use of COAGADEX in routine prophylaxis of bleeding episodes was evaluated in nine children aged less than 12 years of age. The mean age was 7.3 (range 2.6 to 11.9) years. Eight subjects had severe FX deficiency and the other had moderate deficiency. Four subjects were between 0 and 5 years of age and five were between 6 and 11 years of age inclusive. The majority of subjects were Asian (7; 77.8%) and the remainder were Caucasian/White (2; 22.2%). After the first dose of COAGADEX 50 IU/kg, given at a rate not exceeding 3 mL/minute, all subjects underwent a 30-minute post-dose incremental recovery assessment. Routine prophylaxis was started on Day 2 or 3 with unit doses of 40-50 IU/kg and during the first 6 weeks trough levels of Factor X were measured to adjust the dosage regimen to maintain a trough level of at least 5 IU/dL. At the end of the study (at least 6 months and at least 50 exposure days) a repeat 30-minute incremental recovery was performed. A total of 537 (mean 59.7 per subject) prophylactic infusions were administered. The median prophylactic dose per infusion per subject was 39.60 IU/kg (mean 38.76 IU/kg), and ranged from 18.0 to 47.3 IU/kg. Median and mean doses per infusion in the four children less than 6 years of age were both 40.1 IU/kg (95% CI 30.70, 49.57) and in the five children 6 to 11 years of age inclusive, median dose was 39.6 IU/kg and mean dose was 37.7 IU/kg (95% CI: 23.42, 51.91). The median dosing interval for all of the nine children was 3 days (range 2 to 8 days). Investigators' assessment following 6 months of routine prophylaxis was rated excellent in all 9 subjects; excellent was defined as 'no minor or major bleeds occurred during the study period' or 'lower frequency of bleeds than expected, given subject's medical/treatment history'. In addition, 22 infusions were given to treat a bleed, equivalent to 2.1 bleeds per subject per year. One subject had three episodes of epistaxis and the other bleeds were due to trauma or menorrhagia. All bleeds were treated with a single infusion; the median and mean doses per subject were both 31.7 IU/kg (range 24.6 to 38.8 IU/kg) and all recorded efficacy ratings were categorized as 'excellent' i.e. Overt bleed: bleeding stopped within 12 hours with a single dose; Menorrhagic bleed: no additional doses required; Covert bleeds: there were none in this study.
On-demand Treatment and Control of Bleeding Episodes
In a multicenter, open-label, non-randomized clinical trial to evaluate the pharmacokinetics, safety and efficacy of COAGADEX, 16 subjects with moderate to severe hereditary Factor X deficiency (FX:C < 5 IU/dL) received a dose of 25 IU/kg COAGADEX to treat spontaneous, traumatic and menorrhagic bleeding episodes. If hemostasis was not achieved with a single dose of COAGADEX, additional doses could be given until the bleed stopped. Subjects could also continue with treatment after the bleed had stopped to reduce the risk of recurrence of a given bleed. Subjects were aged 12 to 58 years, including 6 pediatric subjects aged 12 to 17 years. Six subjects were male, 10 were female, and 12 were Caucasian.
The efficacy of COAGADEX in treating bleeding episodes was assessed by the subject and/or investigator for each new bleeding episode, using a bleed-specific ordinal rating scale of excellent, good, poor and unassessable for each type of bleed (overt, covert or menorrhagic). Overt bleed: 'excellent' if bleeding stopped within 12 hours with a single dose; 'good' if within 24 hours with ≤2 doses. Covert bleed: 'excellent' if bleeding stopped within 48 hours with 1 or 2 doses; 'good' if within 48 hours with ≤3 doses. Menorrhagic bleed: 'excellent' if ≤2 doses within 48 hours; 'good' if 2 doses over >48 hours. Each bleed was reviewed by a Data Review Committee for its suitability for the efficacy evaluation. Of the 208 bleeding episodes treated with COAGADEX, 187 bleeding episodes in 15 subjects were evaluated for efficacy. Of these 187 bleeding episodes, 79 (42%) occurred spontaneously, 47 (25%) were traumatic and 61 (33%) were menorrhagic. Seventy three (39%) were mucosal in origin, 63 (34%) were joint bleeds, 26 (14%) were muscle bleeds, and 25 (13%) were located elsewhere.
Ninety eight (53%) were major bleeding episodes, and 88 (47%) were minor bleeds (one bleed not assessed). COAGADEX was considered to be good (7%) or excellent (91%) in treating 98% of bleeding episodes. Of the 187 bleeding episodes in the efficacy analysis, a total of 155 bleeds (83%) were treated with one infusion, 28 bleeds (15%) with two infusions, 3 bleeds (2%) with three infusions and 1 bleed (0.5%) with four infusions. The mean dose per infusion and total dose of COAGADEX were 25.4 IU/kg and 30.4 IU/kg, respectively. Four bleeding episodes in two subjects were considered treatment failures.
The recommended dose of 25 IU/kg COAGADEX to treat a bleed was maintained during the study for 14 of the 16 subjects. The other two subjects used doses up to 30 IU/kg and 33 IU/kg.
Perioperative Management of Bleeding
In two multicenter, open-label, non-randomized clinical trials, subjects requiring surgery received a pre-surgical COAGADEX dose to raise plasma Factor X levels to 70-90 IU/dL, followed by post-surgical doses as necessary to maintain plasma Factor X levels at a minimum of 50 IU/dL until the subject was no longer at risk of bleeding due to surgery. The safety and efficacy of COAGADEX for perioperative management was evaluated in five subjects aged 14 to 59 years with mild, moderate or severe hereditary Factor X deficiency, who underwent a total of seven surgical procedures.
For all surgical procedures, COAGADEX was assessed as excellent by the investigator for overall hemostatic control of peri-operative blood loss. Assessment of hemostatic efficacy was based on parameters including: blood loss during surgery, blood transfusion requirements, bleeding episodes and hemoglobin levels. An excellent score required the achievement of outcomes in these parameters similar to patients without a bleeding disorder. For major surgeries, a median of 13 infusions (range 2 to 15 infusions) and a median cumulative dose of 181 IU/kg (range 45 to 210 IU/kg) were required to maintain hemostasis. For minor surgeries, a median of 2.5 infusions (range 1 to 4 infusions) and a median cumulative dose of 89 IU/kg (range 51 to 127 IU/kg) were required to maintain hemostasis.
One subject had insertion of a central venous access device (Portacath) and was given 6 infusions of COAGADEX during 5 days, a total of 2,750 IU (27 IU/kg); there were no bleeding complications or safety concerns.
In a post-marketing registry study, 3 subjects (aged 21 to 30 years) with severe hereditary Factor X deficiency received a total of 10 (mean 3.3) doses of COAGADEX for perioperative hemostatic cover. The median initial (pre-surgery) dose was 44.3 IU/kg (mean 40.6; range 28.6 to 48.9). A median of 4 (range 1 to 5) infusions was administered, with median cumulative dose 110.2 IU/kg (range 28.6 to 171.8). All three subjects achieved excellent hemostatic efficacy outcomes.
16. How is Coagadex supplied
How Supplied
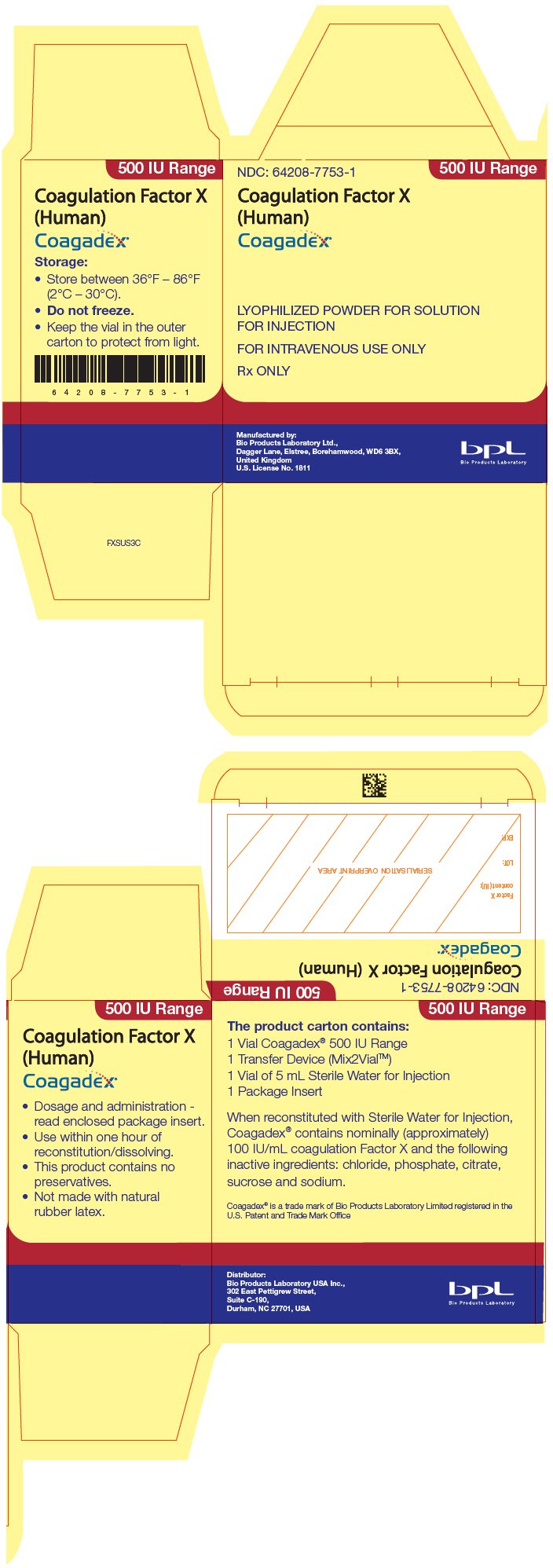
COAGADEX is supplied in single-dose glass vials containing a nominal (approximate) 250 IU or 500 IU (approximately 100 IU/mL after reconstitution) of Factor X activity, packaged with 2.5 mL or 5 mL of Sterile Water for Injection, respectively, and a Mix2Vial transfer device.
The vials are closed with a synthetic rubber stopper. The stopper is not made with natural rubber latex. The stopper is secured with an aluminum overseal with a flip-off polypropylene cap.
| Strength | Kit NDC Number |
|---|---|
| 250 IU Range | 64208-7752-1 |
| 500 IU Range | 64208-7753-1 |
Storage and Handling
- Store COAGADEX in its original package to protect it from light.
- Store the COAGADEX package in a refrigerator or at room temperature at 36°F to 86°F (2°C to 30°C). Do not freeze.
- Do not use COAGADEX or the Sterile Water for Injection after the expiration date printed on the vial and carton labels.
- Use reconstituted COAGADEX within one hour of reconstitution.
- Do not use COAGADEX if the reconstituted solution is cloudy or contains any particles.
17. Patient Counseling Information
- Advise the patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Inform patients to immediately report the following early signs and symptoms of hypersensitivity reactions to their healthcare professional: angioedema, infusion site inflammation (e.g. burning, stinging, erythema), chills, cough, dizziness, fever, flushing, generalized urticaria, headache, hives, hypotension, lethargy, musculoskeletal pains, nausea, pruritus, rash, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing [see Warnings and Precautions (5.1)].
- Inform patients that the development of inhibitors to Factor X is a possible complication of treatment with COAGADEX. Advise the patients to contact their healthcare provider for further treatment and/or assessment if they experience a lack of clinical response to COAGADEX because this may be a manifestation of an inhibitor [see Warnings and Precautions (5.2)].
- Inform patients that COAGADEX is made from human plasma and may contain infectious agents that can cause diseases. While the risk that COAGADEX can transmit an infection has been reduced by screening plasma donors for prior exposure, testing donated plasma, and inactivating or removing certain viruses during manufacturing, patients should report any symptoms that concern them [see Warnings and Precautions (5.3)].
Manufactured by:
Bio Products Laboratory Ltd.,
Dagger Lane, Elstree,
Borehamwood, WD6 3BX,
United Kingdom.
Marketed by:
Kedrion Biopharma, Inc.
Parker Plaza,
400 Kelby Street,
Fort Lee, NJ 07024
United States
U.S. License No: 1811
COAGADEX® is a registered trade mark of Bio Products Laboratory Limited. Mix2Vial™ is a trade mark of West Pharmaceutical services.
Version 7
Patient Information
COAGADEX® (co-ag-a-dex)
Coagulation Factor X (Human)
This Patient Information leaflet summarizes important information about COAGADEX. Please read it carefully before using COAGADEX and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or treatment, and it does not include all of the important information about COAGADEX. If you have any questions about COAGADEX after reading this information, ask your healthcare provider.
What is the most important information I need to know about COAGADEX?
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing COAGADEX so that your treatment will work best for you.
What is COAGADEX?
COAGADEX is a medicine given as an infusion into the vein to replace the clotting factor that is missing in people with Factor X deficiency. Factor X deficiency is an inherited bleeding disorder that prevents blood from clotting normally.
COAGADEX is used to treat, control or reduce bleeding in patients with hereditary Factor X deficiency.
Your healthcare provider may give you COAGADEX when you have surgery.
Who should not use COAGADEX?
You should not use COAGADEX if you are allergic (hypersensitive) to any of the ingredients in COAGADEX.
Tell your healthcare provider if you are pregnant or breast-feeding because COAGADEX may not be right for you.
What should I tell my healthcare provider before I use COAGADEX?
You should tell your healthcare provider if you:
- Have or have had any medical problems
- Have any allergies
- Take any prescription and non-prescription medicines, including over-the-counter medicines, supplements or herbal medicines
- Are breast-feeding. It is not known if COAGADEX passes into your milk and if it can harm your baby
- Are pregnant or planning to become pregnant. It is not known if COAGADEX may harm your unborn baby
- Have been told you have inhibitors to Factor X
How should I use COAGADEX?
You get COAGADEX as an infusion into your vein.
You may infuse COAGADEX at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your healthcare provider or hemophilia treatment center. Many people with coagulation factor deficiencies learn to infuse their treatment by themselves or with the help of a family member or caregiver.
Your healthcare provider will tell you how much COAGADEX to use based on your weight, the severity of your Factor X deficiency, and where you are bleeding from.
You may need blood tests done after using COAGADEX to be sure that the level of Factor X in your blood is high enough to clot your blood.
Call your healthcare provider right away if your bleeding does not stop after using COAGADEX.
What are the possible side-effects of COAGADEX?
You can have an allergic reaction to COAGADEX.
Stop treatment and call your healthcare provider right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash or hives.
Common side effects of COAGADEX are infusion site redness, infusion site pain, tiredness, and back pain.
Your body can also make antibodies, called "inhibitors," against COAGADEX, which may stop COAGADEX from working properly. Your healthcare provider may give you blood tests to check for inhibitors.
These are not all of the possible side effects of COAGADEX. Tell your healthcare provider about any side effects that bother you or do not go away.
What are the COAGADEX dosage strengths?
COAGADEX is packaged with a suitable volume (2.5 mL or 5 mL) of Sterile Water for Injection, and one Mix2Vial transfer device.
COAGADEX comes in two different dosage strengths:
Approximately 250 International Units (IU) – to be reconstituted with 2.5 mL of sterile water.
Approximately 500 International Units (IU) - to be reconstituted with 5 mL of sterile water.
The actual strength will be printed on the vial label and on the box. Once dissolved, the concentrations in these two vials will be the same, 100 IU per mL.
Always check the actual dosage printed on the label to make sure you are using the vial size prescribed by your healthcare provider.
Always check the expiration date printed on the box. Do not use the product after the expiration date printed on the box.
How do I store COAGADEX?
- Keep COAGADEX in its original package to protect it from light.
- Store COAGADEX in a refrigerator (not below 36°F [2°C]) or at room temperature (not to exceed 86°F [30°C]). Do not freeze.
What else should I know about COAGADEX?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use COAGADEX for a condition for which it is not prescribed.
Do not share COAGADEX with other people, even if they have the same symptoms or condition that you have.
For further information or if you have any questions about COAGADEX, please contact Kedrion Biopharma, Inc. at the address below or through US_Medicalinfo@kedrion.com.
Manufactured by:
Bio Products Laboratory Ltd.,
Dagger Lane, Elstree,
Borehamwood, WD6 3BX,
United Kingdom.
Marketed by:
Kedrion Biopharma, Inc.
Parker Plaza,
400 Kelby Street,
Fort Lee, NJ 07024
United States
U.S. License No: 1811
Version 7
Instructions for Use
COAGADEX® (co-ag-a-dex)
Coagulation Factor X (Human)
Important: Do not attempt to give an infusion to yourself unless you have been taught how to by your healthcare provider or hemophilia center.
Always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using COAGADEX. If you are unsure of the procedures, please call your healthcare provider before using.
Your healthcare provider will prescribe the dose and when to use COAGADEX. Contact your healthcare provider right away if you accidentally take more than the prescribed dose.
Your healthcare provider may need to take blood tests from time to time.
Talk to your healthcare provider before traveling. Plan to take enough COAGADEX for your treatment during this time.
Dispose of all materials, including any leftover reconstituted COAGADEX product, in an appropriate container.
How should I dissolve COAGADEX before use?
COAGADEX must be dissolved in the sterile water provided with the product using the provided transfer device called Mix2Vial™.
See below for step-by-step instructions for reconstituting COAGADEX.
|
| Step 1
|
| Step 5
|
|
| Step 3
|
| Step 6
|
|
| Step 4
|
| Step 8
|
If you have to use more than one vial of COAGADEX to make up your dose, repeat steps 1 to 5 to reconstitute each vial and draw up all of the solution into one syringe for your infusion. You must use a new Mix2Vial to draw the contents of each vial up into the plastic syringe.
How do I infuse COAGADEX?
Inspect the solution before infusion. Do not use if there are any particles in the syringe, or if the solution is cloudy, or if a gel or clot forms.
Do not add the solution to any other fluids and do not mix it together with any other medicine.
To infuse the medicine:
- Attach a suitable needle to the syringe containing COAGADEX solution.
- Inject the dose at a suggested rate of 10 mL/min, but no more than 20 mL/min into your vein.
After infusing the medicine:
After infusion, safely dispose of the needle, tubing, syringe, any unused COAGADEX and other waste materials. Record the infusion in your infusion tracker or calendar.
Important: Contact your healthcare provider or local hemophilia treatment center if you experience any problems.
COAGADEX® is a registered trade mark of Bio Products Laboratory Limited. Mix2Vial™ is a trade mark of West Pharmaceutical services.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Bio Products Laboratory Ltd., Dagger Lane, Elstree, Borehamwood, WD6 BX, United Kingdom.
U.S. License No: 1811
Revised 03/2023
Version 7
PRINCIPAL DISPLAY PANEL - 250 IU Kit Carton
NDC: 64208-7752-1
250 IU Range
Coagulation Factor X
(Human)
Coagadex®
LYOPHILIZED POWDER FOR SOLUTION
FOR INJECTION
FOR INTRAVENOUS USE ONLY
Rx ONLY
Manufactured by:
Bio Products Laboratory Ltd.,
Dagger Lane, Elstree, Borehamwood, WD6 3BX,
United Kingdom
U.S. License No. 1811
Bio Products Laboratory
PRINCIPAL DISPLAY PANEL - 250 IU Vial Label
Coagulation Factor X
(Human)
Coagadex®
250 IU Range
- FOR INTRAVENOUS USE ONLY.
- Read enclosed package insert before use.
- Rx only.
- Store between 36°F – 86°F (2°C – 30°C).
- DO NOT FREEZE.
NDC: 64208-7754-1
FXRUS4L

PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
Sterile Water
for Injection
2.5 mL
Store between 36°F – 86°F (2°C – 30°C).
Do not freeze.
Do not use unless clear.
NDC: 64208-7755-1
Bio Products Laboratory Ltd., Elstree,
Borehamwood, WD6 3BX, United Kingdom

PRINCIPAL DISPLAY PANEL - 500 IU Kit Carton
NDC: 64208-7753-1
500 IU Range
Coagulation Factor X
(Human)
Coagadex®
LYOPHILIZED POWDER FOR SOLUTION
FOR INJECTION
FOR INTRAVENOUS USE ONLY
Rx ONLY
Manufactured by:
Bio Products Laboratory Ltd.,
Dagger Lane, Elstree, Borehamwood, WD6 3BX,
United Kingdom
U.S. License No. 1811
Bio Products Laboratory
| COAGADEX
coagulation factor x human kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| COAGADEX
coagulation factor x human kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bio Products Laboratory Limited (216845337) |
More about Coagadex (coagulation factor x)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous coagulation modifiers
- Breastfeeding
- En español